109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

一个单剂量和多剂量研究,以评估固定剂量的依巴司韦/艾尔巴韦在健康的中国参与者中的药代动力学
Authors Li H, Yang Z, Zhang S, Xu L, Wei Y, Jiang J, Caro L, Feng HP, McCrea JB, Li M, Xie S, Wang J, Zhao XM, Mu S
Received 25 July 2019
Accepted for publication 11 December 2019
Published 11 February 2020 Volume 2020:12 Pages 1—11
DOI https://doi.org/10.2147/CPAA.S224662
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Professor Arthur Frankel
Purpose: The burden of hepatitis C virus infection is particularly high in Asian countries, and new treatments are urgently needed. The purpose of this study was to characterize the pharmacokinetics (PK) and safety of the fixed-dose combination tablet of elbasvir/grazoprevir in healthy Chinese participants.
Patient and Methods: In this Phase I, single-site, open-label, 3-period study in healthy Chinese adults, participants received a single tablet of elbasvir 50 mg/grazoprevir 100 mg, followed by blood sampling for up to 96 hrs (http://www.chinadrugtrials.org.cn/CTR20160034; Protocol PN071). Participants then received 1 tablet daily for 10 days, followed by a minimum 10-day washout, after which participants received a single dose of 2 tablets (elbasvir 100 mg/grazoprevir 200 mg). Elbasvir and grazoprevir PK were assessed following single and multiple doses. Safety and tolerability were also evaluated.
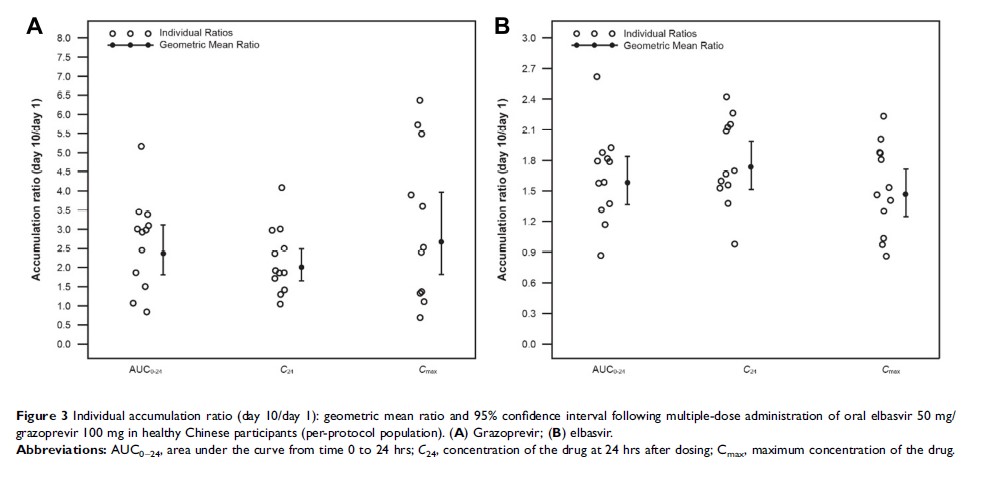
Results: Twelve participants (50% male) were enrolled in and completed the study. Following single-dose oral administration of elbasvir 50 mg/grazoprevir 100 mg or elbasvir 100 mg/grazoprevir 200 mg, the median Tmax was 3– 4 hrs and elimination half-life was 18 hrs (elbasvir) and 30 hrs (grazoprevir). Multiple-dose administration resulted in AUC0– 24 accumulation ratios of 1.58 (elbasvir) and 2.35 (grazoprevir). Both elbasvir 50 mg/grazoprevir 100 mg and 100 mg/200 mg regimens were generally well tolerated.
Conclusion: Single-dose administration of elbasvir 50 mg/grazoprevir 100 mg or 100 mg/200 mg and once-daily administration of elbasvir 50 mg/grazoprevir 100 mg for 10 days has been adequately characterized, with PK values within the expected range, and was generally well tolerated in healthy Chinese male and female participants.
Keywords: elbasvir, grazoprevir, healthy volunteers, hepatitis C virus, pharmacokinetics