109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
SDF-1/CXCR4 在脑动静脉畸形的血管形成和重塑中的作用
Authors Wang L, Guo S, Zhang N, Tao Y, Zhang H, Qi T, Liang F, Huang Z
Received 29 April 2015
Accepted for publication 12 June 2015
Published 1 September 2015 Volume 2015:11 Pages 1337—1344
DOI http://dx.doi.org/10.2147/TCRM.S87590
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Chung-Liang Lai
Peer reviewer comments 3
Editor who approved publication: Professor Deyun Wang
Background: Cerebral arteriovenous malformation (AVM) involves the vasculogenesis of cerebral blood vessels and can cause severe intracranial hemorrhage. Stromal cell-derived factor-1 (SDF-1) and its receptor, CXCR4, are believed to exert multiple physiological functions including angiogenesis. Thus, we investigated the role of SDF-1/CXCR4 in the vasculogenesis of cerebral AVM.
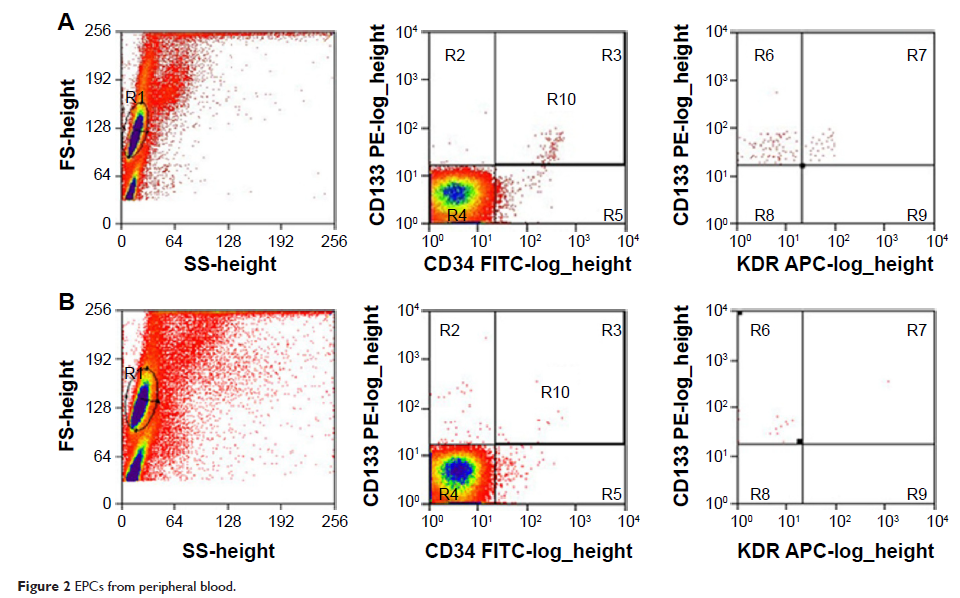
Methods: Brain AVM lesions from surgical resections were analyzed for the expression of SDF-1, CXCR4, VEGF-A, and HIF-1 by using immunohistochemical staining. Flow cytometry was used to quantify the level of circulating endothelial progenitor cells (EPCs). Further, in an animal study, chronic cerebral hypoperfusion model rats were analyzed for the expression of SDF-1 and HIF-1. CXCR4 antagonist, AMD3100, was also used to detect its effects on cerebral vasculogenesis and SDF-1 expression.
Results: Large amounts of CXCR4-positive CD45+ cells were found in brain AVM lesion blood vessel walls, which also have higher SDF-1 expression. Cerebral AVM patients also had higher level of EPCs and SDF-1. In chronic cerebral hypoperfusion rats, SDF-1, HIF-1, and CD45 expressions were elevated. The application of AMD3100 effectively suppressed angiogenesis and infiltration of CXCR4-positive CD45+ cells in hypoperfusion rats compared to controls.
Conclusion: The SDF-1/CXCR4 axis plays an important role in the vasculogenesis and migration of inflammatory cells in cerebral AVM lesions, possibly via the recruitment of bone marrow EPCs.
Keywords: cerebral arteriovenous malformation, SDF-1/CXCR4, chronic cerebral hypoperfusion, endothelial progenitor cells
Methods: Brain AVM lesions from surgical resections were analyzed for the expression of SDF-1, CXCR4, VEGF-A, and HIF-1 by using immunohistochemical staining. Flow cytometry was used to quantify the level of circulating endothelial progenitor cells (EPCs). Further, in an animal study, chronic cerebral hypoperfusion model rats were analyzed for the expression of SDF-1 and HIF-1. CXCR4 antagonist, AMD3100, was also used to detect its effects on cerebral vasculogenesis and SDF-1 expression.
Results: Large amounts of CXCR4-positive CD45+ cells were found in brain AVM lesion blood vessel walls, which also have higher SDF-1 expression. Cerebral AVM patients also had higher level of EPCs and SDF-1. In chronic cerebral hypoperfusion rats, SDF-1, HIF-1, and CD45 expressions were elevated. The application of AMD3100 effectively suppressed angiogenesis and infiltration of CXCR4-positive CD45+ cells in hypoperfusion rats compared to controls.
Conclusion: The SDF-1/CXCR4 axis plays an important role in the vasculogenesis and migration of inflammatory cells in cerebral AVM lesions, possibly via the recruitment of bone marrow EPCs.
Keywords: cerebral arteriovenous malformation, SDF-1/CXCR4, chronic cerebral hypoperfusion, endothelial progenitor cells