109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

贝伐单抗 (Bevacizumab) 联合化疗在晚期结直肠癌三线以上治疗中的应用价值
Authors Yang Q, Yin CX, Liao FX, Huang YY, He WZ, Jiang C, Guo GF, Zhang B, Xia LP
Received 16 May 2015
Accepted for publication 23 June 2015
Published 1 September 2015 Volume 2015:8 Pages 2407—2413
DOI http://dx.doi.org/10.2147/OTT.S88679
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 4
Editor who approved publication: Professor Daniele Santini
Background: Currently available third- or later-line therapy for metastatic colorectal cancer (mCRC) is limited in its efficacy, with a weak survival benefit in patients who progressed after two or more lines of standard therapy. Our retrospective study aimed to explore the value of bevacizumab plus chemotherapy in this setting.
Methods: Patients with mCRC who received fluoropyrimidine, oxaliplatin, and irinotecan as first- and second-line chemotherapy were selected for inclusion. Treatment consisted of bevacizumab plus chemotherapy. Chemotherapy consisted mainly of oxaliplatin, irinotecan, and fluoropyrimidine.
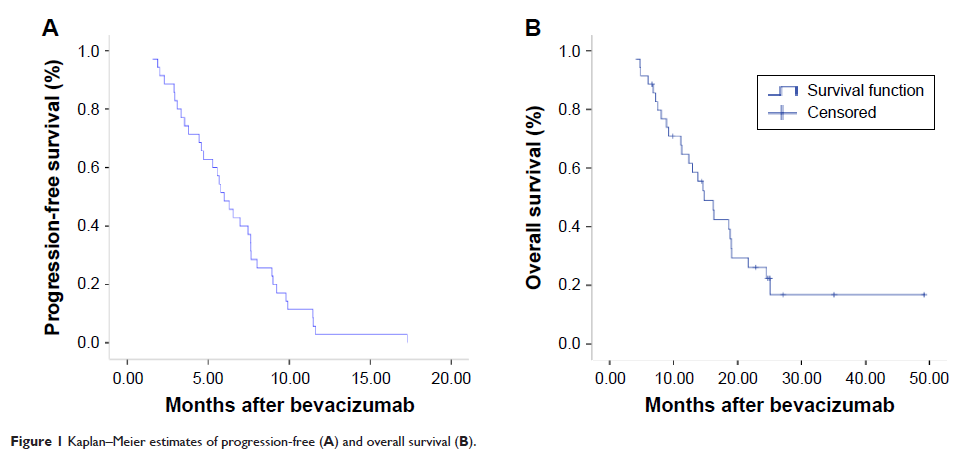
Results: Between February 2010 and December 2012, 35 consecutive patients with mCRC were treated with bevacizumab plus chemotherapy as a third- or later-line treatment. No complete responses, seven partial responses (20%), 22 stable disease responses (62.9%), and six progressive disease responses (17.1%) were obtained, producing an objective response rate of 20% and a disease control rate of 82.9%. With a median follow-up of 11.3 months (range: 0.7–48.0 months), the median progression-free survival was 5.98 months (95% confidence interval: 4.76–7.2 months), and the median overall survival was 14.77 months (95% confidence interval: 11.45–18.1 months). In the univariate analysis, patients with a primary colon tumor might have had a longer overall survival than patients with a primary rectal tumor (18.8 months vs 11.1 months, respectively; P =0.037). Common chemotherapy-related toxicities were nausea/vomiting (48.6%), fatigue (34.3%), leucopenia (40%), neutropenia (42.9%), and anemia (42.9%), with one patient with grade 3 neutropenia, and two patients with grade 3 thrombocytopenia. The common bevacizumab-associated toxicity was hypertension (31.4%). None of the patients discontinued therapy or died because of bevacizumab-associated toxicities.
Conclusion: Our data showed that adding bevacizumab to third- or later-line therapy might lead to tumor control and improved survival in heavily pretreated mCRC patients. In addition, preliminary data suggested that primary colon cancer was more likely to benefit from bevacizumab-containing regimens. Toxicities were acceptable, and no new toxicity was identified. Further studies are needed to validate these findings.
Keywords: bevacizumab,chemotherapy, metastatic colorectal cancer