109229
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

对 KPNB1 的遏制可通过调节 E2F1 来抑制增殖并促进慢性粒细胞白血病细胞凋亡
Authors Wang T, Huang Z, Huang N, Peng Y, Gao M, Wang X, Feng W
Received 28 March 2019
Accepted for publication 14 November 2019
Published 2 December 2019 Volume 2019:12 Pages 10455—10467
DOI https://doi.org/10.2147/OTT.S210048
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Dr Carlos E Vigil
Background: Karyopherin-β1 (KPNB1) belongs to the karyopherin superfamily, which functions as shuttling proteins from the cytoplasm to nuclear. A high level of KPNB1 has been reported in various cancers which promotes cell proliferation and inhibits apoptosis. However, the role of KPNB1 in chronic myeloid leukemia (CML) remains uncertain.
Methods: Expression level of KPNB1 in CML patient samples and cell lines was analyzed by Western blotting. The proliferation assays and colony formation assay were used to study the CML cell proliferation when KPNB1 knockdown in vitro. Next, Western blotting was used to evaluate the effects of KPNB1 on E2F1 and other cell cycle regulators. Then, the location of E2F1 was detected by immunofluorescence. Finally, flow cytometry was used to detect the effect of KPNB1 inhibitor importazole (IPZ) on CML cells.
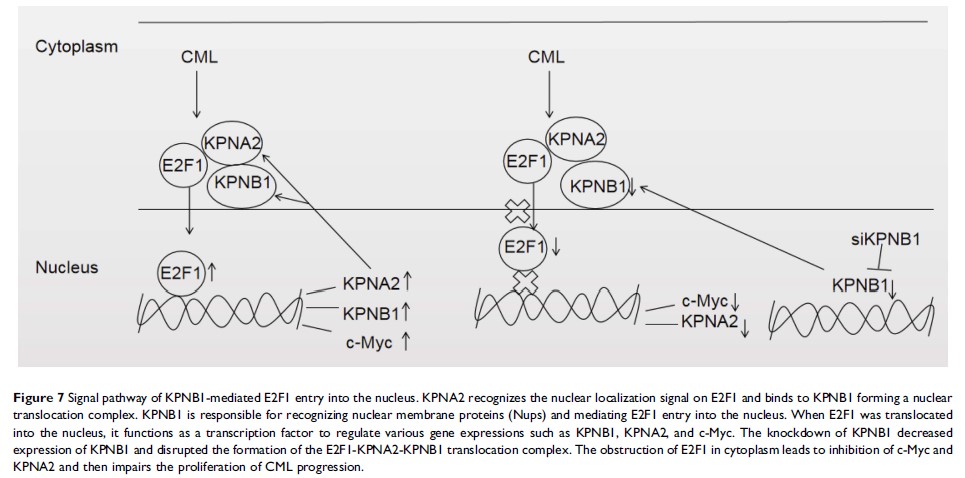
Results: In this study, we firstly showed that KPNB1 is over-expressed in CML cells. Targeting KPNB1 with small interfering RNA (siRNA) and IPZ reduced proliferation and induced apoptosis of CML cells. The underlying mechanisms were also investigated that E2F1 nuclear transport was blocked after inhibiting KPNB1 with siRNA, suggesting KPNB1 over-expression mediates the excessive nuclear transport of E2F1 in CML cells. Moreover, the expression of the E2F1 targeted molecule such as c-Myc and KPNA2 was markedly reduced. The IPZ arrested CML cells at G2/M phase and induced cell apoptosis.
Conclusion: In summary, our results clearly showed that KPNB1 is over-expressed in CML cells and mediates the translocation of E2F1 into the nucleus of CML cells, thereby inhibition of KPNB1 reduced proliferation and induced apoptosis of CML cells which provides new insights for targeted CML therapies.
Keywords: CML, KPNB1, E2F1, c-Myc, IPZ, subcellular location