109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
RGD 修饰的脂质体可提高阿克拉霉素 A (aclacinomycin A) 的给药效率: 对他们在肺癌治疗中的效果评价
Authors Feng C, Li X, Dong C, Zhang X, Zhang X, Gao Y
Received 2 April 2015
Accepted for publication 5 May 2015
Published 11 August 2015 Volume 2015:9 Pages 4613—4620
DOI http://dx.doi.org/10.2147/DDDT.S85993
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Professor Wei Duan
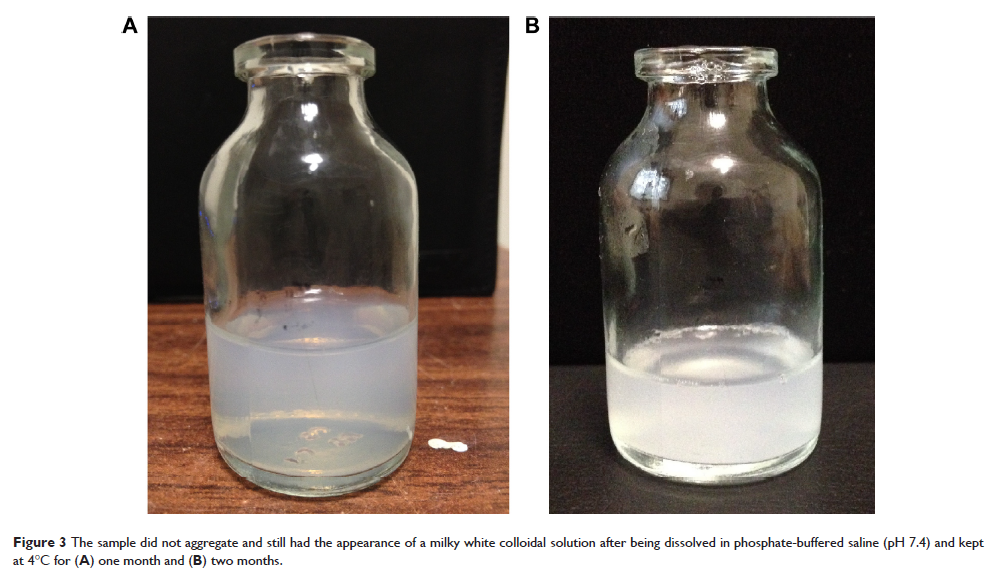
Abstract: In this study, long-circulating Arg-Gly-Asp (RGD)-modified aclacinomycin A (ACM) liposomes were prepared by thin film hydration method. Their morphology, particle size, encapsulation efficiency, and in vitro release were investigated. The RGD-ACM liposomes was about 160 nm in size and had the visual appearance of a yellowish suspension. The zeta potential was -22.2 mV and the encapsulation efficiency was more than 93%. The drug-release behavior of the RGD-ACM liposomes showed a biphasic pattern, with an initial burst release and followed by sustained release at a constant rate. After being dissolved in phosphate-buffered saline (pH 7.4) and kept at 4°C for one month, the liposomes did not aggregate and still had the appearance of a milky white colloidal solution. In a pharmacokinetic study, rats treated with RGD-ACM liposomes showed slightly higher plasma concentrations than those treated with ACM liposomes. Maximum plasma concentrations of RGD-ACM liposomes and ACM liposomes were 4,532 and 3,425 ng/mL, respectively. RGD-ACM liposomes had a higher AUC0–∞ (1.54-fold), mean residence time (2.09-fold), and elimination half-life (1.2-fold) when compared with ACM liposomes. In an in vivo study in mice, both types of liposomes inhibited growth of human lung adenocarcinoma (A549) cells and markedly decreased tumor size when compared with the control group. There were no obvious pathological tissue changes in any of the treatment groups. Our results indicate that RGD-modified ACM liposomes have a better antitumor effect in vivo than their unmodified counterparts.
Keywords: RGD, aclacinomycin A, long-circulating liposomes, pharmacokinetic, tumor inhibition
Keywords: RGD, aclacinomycin A, long-circulating liposomes, pharmacokinetic, tumor inhibition