109229
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
一种新型的抗水解亲脂性叶酸衍生可稳定靶向脂质体的体内传递
Authors Huang Y, Yang T, Zhang W, Lu Y, Ye P, Yang G, Li B, Qi S, Liu Y, He X, Lee RJ, Xu C, Xiang G
Published Date September 2014 Volume 2014:9(1) Pages 4581—4595
DOI http://dx.doi.org/10.2147/IJN.S69115
Received 10 June 2014, Accepted 10 July 2014, Published 29 September 2014
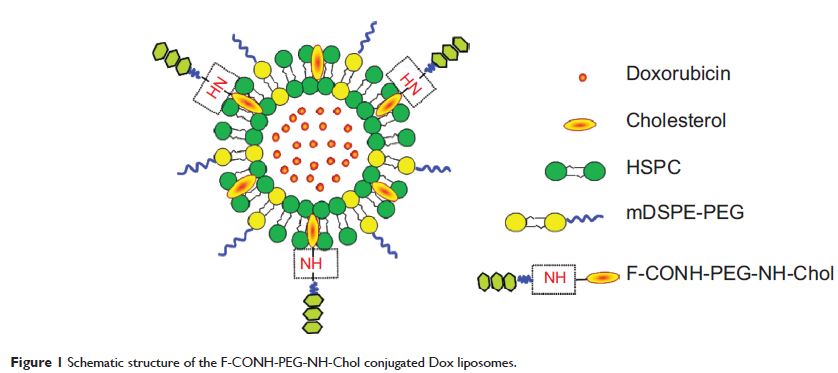
Abstract: Instability of targeting ligand is a roadblock towards successful development of folate targeted liposomes. Folate ligands have been linked to polyethylene glycol (PEG) and cholesterol by an amide bond to form folate-CONH-PEG-CONH-Cholesterol (F-CONH-PEG-CONH-Chol), which is subject to hydrolysis. To increase the stability of folate ligands and promote the long circulation and targeting effects, we synthesized a chemically stable lipophilic folate derivative, folate-CONH-PEG-NH-Cholesterol (F-CONH-PEG-NH-Chol), where the amide bond was replaced by a C-N bond, to deliver liposomal doxorubicin (Dox). Its physical stability, cellular uptake, cellular toxicity, pharmacokinetics, distribution, anti-tumor efficacy, and cardiac toxicity were investigated. Our results indicate that F-CONH-PEG-NH-Chol conjugated liposomes are taken up selectively by folate receptor-positive HeLa and KB cells. Compared with F-CONH-PEG-CONH-Chol with two carbonate linkages, F-CONH-PEG-NH-Chol better retained its drug entrapment efficiency and folate receptor-targeting activity during prolonged circulation. F-CONH-PEG-NH-Chol thus represents a physically stable and effective ligand for delivering folate receptor-targeted liposomes, with prolonged circulation time and efficient tissue distribution, as well as higher efficacy and less cardiac toxicity. Collectively, these results suggest that this novel conjugate can serve as a promising derivative for the delivery of anti-tumor therapeutic agents.
Keywords: liposome, doxorubicin, folate ligand, FR-targeting, stability
Keywords: liposome, doxorubicin, folate ligand, FR-targeting, stability