109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
治疗特发性肺纤维化的酪氨酸激酶抑制剂候选药物 KBP-7018 的临床前体内和体外的药代动力学特征研究
Authors Huang Z, Li H, Zhang Q, Tan X, Lu F, Liu H, Li S
Received 17 February 2015
Accepted for publication 18 March 2015
Published 5 August 2015 Volume 2015:9 Pages 4319—4328
DOI http://dx.doi.org/10.2147/DDDT.S83055
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
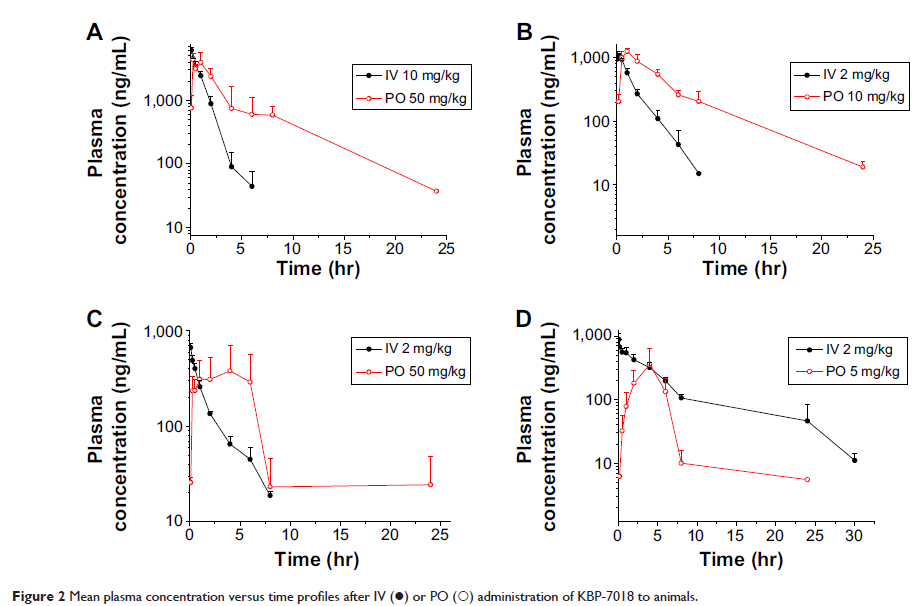
Abstract: KBP-7018 is a novel selective tyrosine kinase inhibitor with potential for the treatment of idiopathic pulmonary fibrosis. The objective of this study was to characterize the preclinical pharmacokinetics of KBP-7018 in vitro and in vivo, and then to assess the likelihood of developing KBP-7018 as a clinical candidate. The systemic clearance (CL) of KBP-7018 was relatively low in rodents and monkeys with a value of less than 30% of hepatic blood flow, while it was high in dogs. The steady-state volume of distribution (V ss) ranged from 1.51 L/kg to 4.65 L/kg across the species tested. The maximum concentration (C max) of KBP-7018 occurred at 0.25–6 hours after oral dosing, and the bioavailability was moderate (21%–68%). The human CL (~20% of hepatic blood flow) and V ss (1.6–5.3 L/kg) were predicted by allometric scaling method and together with the other modeling methods indicated low metabolism and acceptable half-time (4.8–19.3 hours) in vivo. Overall, the preclinical data make it amenable to further oral solid dosage from design for the upcoming Phase I trials in human.
Keywords: idiopathic pulmonary fibrosis, tyrosine kinase inhibitor, pharmacokinetics, KBP-7018
Keywords: idiopathic pulmonary fibrosis, tyrosine kinase inhibitor, pharmacokinetics, KBP-7018