108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

在禁食和进食条件下健康的中国志愿者服用含美力信 10 mg 和氟喷托醇 0.5 mg 的固定剂量组合片剂的生物等效性研究
Authors Wu L, Xu C, Wu G, Zhou H, Lv D, Zhai Y, Huang Y, Tang W, Li F, Shentu J
Received 11 March 2019
Accepted for publication 29 August 2019
Published 19 September 2019 Volume 2019:13 Pages 3331—3342
DOI https://doi.org/10.2147/DDDT.S207561
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Melinda Thomas
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Cristiana Tanase
Purpose: A fixed-dose combination (FDC) tablet of melitracen/flupentixol has been widely used for depression. The purpose of this study was to assess the safety profile and the relative bioavailability of two FDC products containing 10 mg melitracen and 0.5 mg flupentixol from two different manufacturers, in order to acquire adequate pharmacokinetic evidence for registration approval of the test formulation.
Methods: The study was designed as a single-dose, randomized, open-label, 2-period crossover study under fasted or fed conditions in healthy Chinese subjects. Twenty-four subjects (16 men and 8 women) were selected for fasted study, and another 24 cases (16 men and 8 women) were in fed study. Each subject was randomized at the beginning to receive either a single dose of the reference FDC or the test FDC tablet during the first period. Following two-week washout period, all subjects received the alternate formulation during the second period. Blood samples were collected up to 144 hrs after administration. Pharmacokinetic parameters, including Cmax, Tmax, AUC0-t, AUC0-∞, t½, CL/F, and Vd/F were acquired based on the time versus concentration profiles. Then, the geometric mean ratios (GMR) and corresponding 90% CIs were calculated for the determination of bioequivalence analysis. Safety assessment included changes in vital signs and laboratory tests, physical examination findings, and incidence or reports of adverse events (AEs).
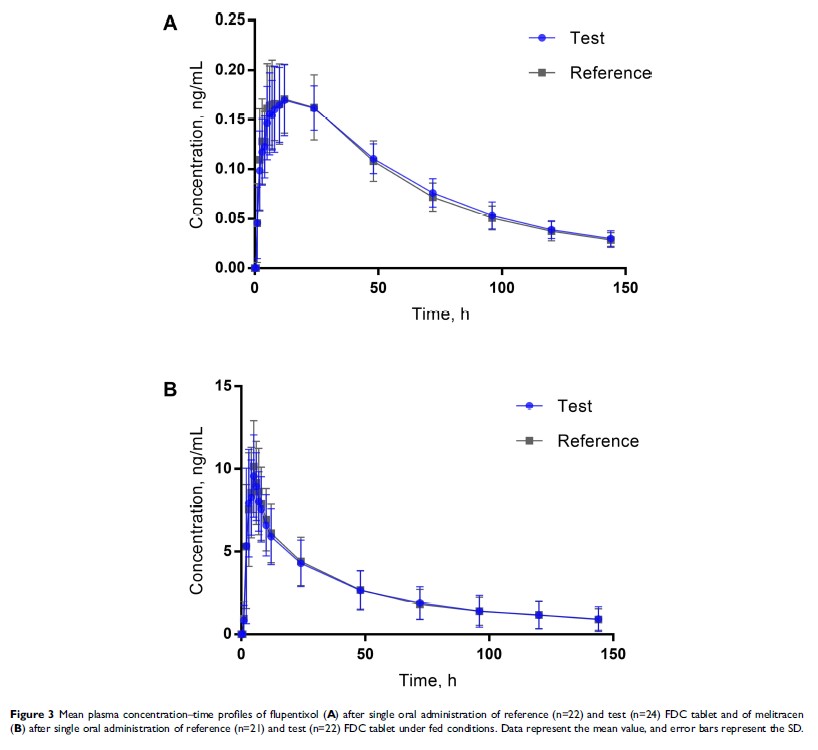
Results: The present study has clearly indicated the test and the reference FDC products are bioequivalent in terms of rate and extent of drug absorption. GMR of Cmax, AUC0–t, and AUC0-∞ for both flupentixol and melitracen between the two formulation FDC products, and corresponding 90% CIs, were all within the range of 80% to 125% under fasted or fed conditions. Both the test and the reference FDC products indicated good tolerance in all volunteers. Chinese Clinical Trials Registry identifier: CTR20171256.
Keywords: bioequivalence, melitracen, flupentixol, pharmacokinetics, fixed-dose combination tablet