109229
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
TNYL 肽官能壳聚糖-g-硬脂酸酯偶联胶束用于肿瘤特异性靶向
Authors Chen FY, Yan JJ, Yi HX, Hu FQ, Du YZ, Yuan H, You J, Zhao MD
Published Date September 2014 Volume 2014:9(1) Pages 4597—4608
DOI http://dx.doi.org/10.2147/IJN.S69572
Received 17 June 2014, Accepted 24 July 2014, Published 26 September 2014
Approved for publication by Professor Thomas J. Webster
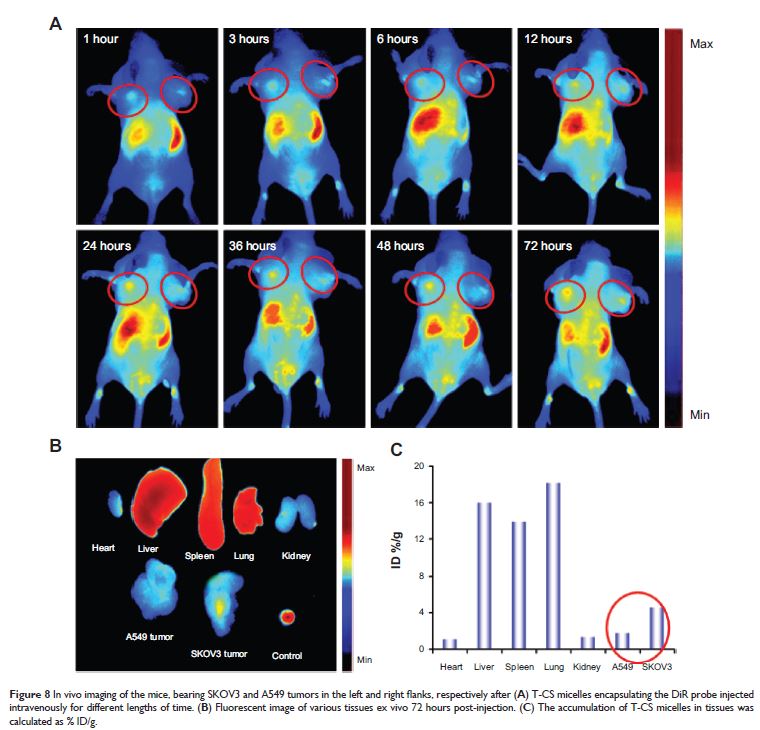
Abstract: Nowadays, a real challenge in cancer therapy is to design drug delivery systems that can achieve high concentrations of drugs at the target site for improved therapeutic effect with reduced side effects. In this research, we designed and synthesized a homing peptide-(TNYLFSPNGPIA, TNYL) modified chitosan-g-stearate (CS) polymer micelle (named T-CS) for targeting delivery. The peptide displayed specific binding affinity to EphB4 which is a member of the Eph family of receptor tyrosine protein kinases. The amphiphilic polymer T-CS can gather into micelles by themselves in an aqueous environment with a low critical micelle concentration value (91.2 µg/L) and nano-scaled size (82.1±2.8 nm). The drug encapsulation efficiency reached 86.43% after loading the hydrophobic drug doxorubicin (DOX). The cytotoxicity of T-CS/DOX against SKOV3 cells was enhanced by approximately 2.3-fold when compared with CS/DOX. The quantitative and qualitative analysis for cellular uptake indicated that TNYL modification can markedly increase cellular internalization in the EphB4-overexpressing SKOV3 cell line, especially with a short incubation time. It is interesting that relatively higher uptake of the T-CS/DOX micelles by SKOV3 cells (positive-EphB4) than A549 cells (negative-EphB4) was observed when the two cells were co-incubated. Furthermore, in vivo distribution experiment using a bilateral-tumor model showed that there was more fluorescence accumulation in the SKOV3 tumor than in the A549 tumor over the whole experiment. These results suggest that TNYL-modified CS micelles may be promising drug carriers as targeting therapy for the EphB4-overexpressing tumor.
Keywords: chitosan-g-stearate, polymeric micelles, TNYL, active targeting, antitumor activity
Keywords: chitosan-g-stearate, polymeric micelles, TNYL, active targeting, antitumor activity