109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
评估维拉佐酮 (Vilazodone) 用于抑郁症治疗的疗效和安全性
Authors Zhang XF, Wu L, Wan DJ, Liu RZ, Dong Z, Chen M, Yu SY
Received 5 May 2015
Accepted for publication 2 July 2015
Published 4 August 2015 Volume 2015:11 Pages 1957—1965
DOI http://dx.doi.org/10.2147/NDT.S87968
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Ryouhei Ishii
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Purpose: Vilazodone is a novel serotonin (5-HT)-reuptake inhibitor and 5-HT1A partial agonist that was recently developed for the treatment of major depressive disorder (MDD). We conducted a meta-analysis and systematic review to better evaluate the efficacy and safety of vilazodone.
Materials and methods: We performed a thorough literature search to identify all randomized double-blind placebo-controlled trials that were designed to investigate the efficacy of vilazodone for the treatment of MDD, and that were published in electronic databases, including Medline, Embase, and the Cochrane Central Register of Controlled Trials. A manual search was also conducted to investigate the relevant references of the retrieved studies. Subsequently, we conducted a meta-analysis and systematic literature review.
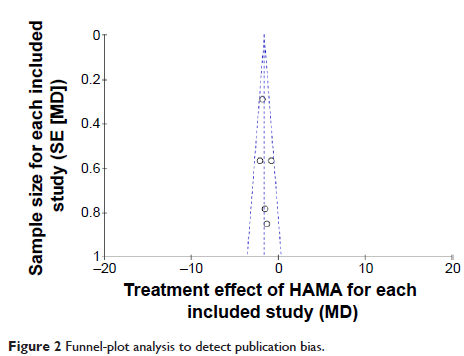
Results: A total of five randomized controlled trials were finally included, involving 1,200 patients with vilazodone and 1,193 patients with placebo. The primary efficacy end point of the Montgomery–Åsberg Depression Rating Scale (standardized mean difference -3.58, 95% confidence interval -4.59 to -2.56; P <0.00001), and the key secondary efficacy end points (Clinical Global Impression - Severity scale, Clinical Global Impression - Improvement scale, and Hamilton Anxiety Rating Scale) indicated that vilazodone was more effective than placebo. Most common adverse events, including diarrhea and nausea, were evaluated, and safety assessments indicated that vilazodone was well tolerated (diarrhea odds ratio 3.54, 95% confidence interval 2.81–4.45; P <0.00001; nausea odds ratio 3.85, 95% confidence interval 3.00–4.96; P <0.00001; discontinuations due to adverse events odds ratio 2.71, 95% confidence interval 1.81–4.05; P <0.00001).
Conclusion: Our findings indicate that the novel antidepressant vilazodone is effective and safe for MDD, with a low occurrence of side effects. It offers promise as an effective oral drug for the treatment of MDD, with a balance of efficacy and tolerability.
Keywords: vilazodone, major depression, systematic review, antidepressant, randomized controlled trial
Materials and methods: We performed a thorough literature search to identify all randomized double-blind placebo-controlled trials that were designed to investigate the efficacy of vilazodone for the treatment of MDD, and that were published in electronic databases, including Medline, Embase, and the Cochrane Central Register of Controlled Trials. A manual search was also conducted to investigate the relevant references of the retrieved studies. Subsequently, we conducted a meta-analysis and systematic literature review.
Results: A total of five randomized controlled trials were finally included, involving 1,200 patients with vilazodone and 1,193 patients with placebo. The primary efficacy end point of the Montgomery–Åsberg Depression Rating Scale (standardized mean difference -3.58, 95% confidence interval -4.59 to -2.56; P <0.00001), and the key secondary efficacy end points (Clinical Global Impression - Severity scale, Clinical Global Impression - Improvement scale, and Hamilton Anxiety Rating Scale) indicated that vilazodone was more effective than placebo. Most common adverse events, including diarrhea and nausea, were evaluated, and safety assessments indicated that vilazodone was well tolerated (diarrhea odds ratio 3.54, 95% confidence interval 2.81–4.45; P <0.00001; nausea odds ratio 3.85, 95% confidence interval 3.00–4.96; P <0.00001; discontinuations due to adverse events odds ratio 2.71, 95% confidence interval 1.81–4.05; P <0.00001).
Conclusion: Our findings indicate that the novel antidepressant vilazodone is effective and safe for MDD, with a low occurrence of side effects. It offers promise as an effective oral drug for the treatment of MDD, with a balance of efficacy and tolerability.
Keywords: vilazodone, major depression, systematic review, antidepressant, randomized controlled trial