108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

表观遗传学机制介导 miR-125a/BRMS1 轴以调节胃癌的侵袭和转移
Authors Xiong J, Tu Y, Feng Z, Li D, Yang Z, Huang Q, Li Z, Cao Y, Jie Z
Received 28 March 2019
Accepted for publication 17 August 2019
Published 12 September 2019 Volume 2019:12 Pages 7513—7525
DOI https://doi.org/10.2147/OTT.S210376
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Ms Aruna Narula
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Purpose: Altered expression of breast cancer metastasis suppressor 1 (BRMS1), is a tumor suppressor, which is found in many types of cancers, including gastric cancer (GC), but the mechanism by which BRMS1 inhibits invasion and metastasis in GC is unknown. The aim of the study was to investigate the molecular mechanisms of miR-125a/BRMS1 in GC.
Materials and methods: The expression of BRMS1 and miR-125a were detected by quantitative real-time PCR (qRT-PCR) and analyzed by bioinformatics. BSP and MSP were used to detecte the methylation status of miR-125a and BRMS1 which was treated by 5-Aza or not. Western Blot and qRT-PCR were used to analyze the expression of BRMS1 and EZH2. Transwell was performed to explore the invasion and metastasis ability of GC cells. The nude mice were used for the tumor formation assay.
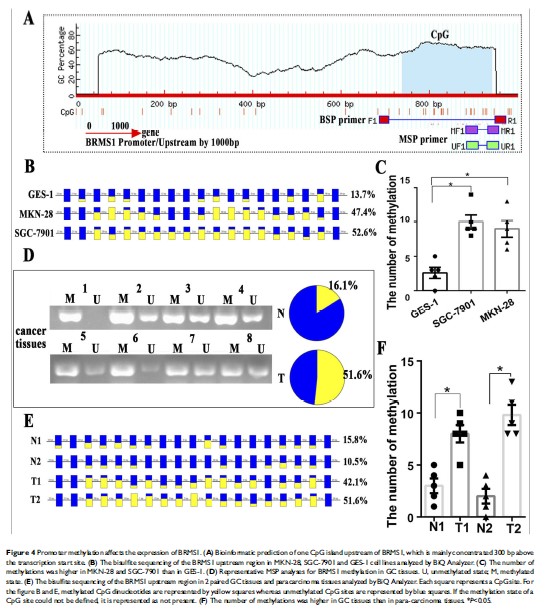
Results: BRMS1 may be regulated by copy number variation (CNV), methylation and miR-125a-5p. As one of the essential components of PRC2, EZH2 is an important regulatory factor resulting in the low expression of miR-125a. An epigenetic mechanism mediates the miR-125a/BRMS1 axis to inhibit the invasion and metastasis of GC cells. In vivo experiments, it is also showed that BRMS1 is involved in invasion and metastasis but not the proliferation in GC.
Conclusion: These studies shed light on the mechanism of BRMS1 inhibition of GC invasion and metastasis and the development of new drugs targeting the miR-125a/BRMS1 axis, which will be a promising therapeutic strategy for GC and other human cancers.
Keywords: gastric cancer, miR-125a/BRMS1 axis, epigenetic, EZH2, invasion and metastasis