108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

冬凌草甲素对结肠癌的抑制作用是通过体内外 TGF-β1/Smads-PAI-1 信号通路的失活来介导的
Authors Bu HQ, Shen F, Cui J
Received 22 June 2019
Accepted for publication 27 August 2019
Published 11 September 2019 Volume 2019:12 Pages 7467—7476
DOI https://doi.org/10.2147/OTT.S220401
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Ms Shreya Arora
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Background: Oridonin, the main active component of Rabdosia rubescens, has been demonstrated to have anti-tumor effect on all kinds of cancer cells through various mechanisms and it has shown antitumor activity in some tumors partially via the suppression of TGF-β/Smads signaling pathway. The aim of this study was to explore the anticancer effect of oridonin on human colon carcinoma and underlying mechanism in vitro and vivo.
Methods: CCK-8 assay was employed to assess cell viability. The key target genes and proteins involved in TGF-β/Smads pathway was detected by RT-PCR, Western blotting and immunohistochemistry. The orthotopic transplantation tumor model of colon cance LOVO cell was introduced to detect anti-cancer effects in vivo.
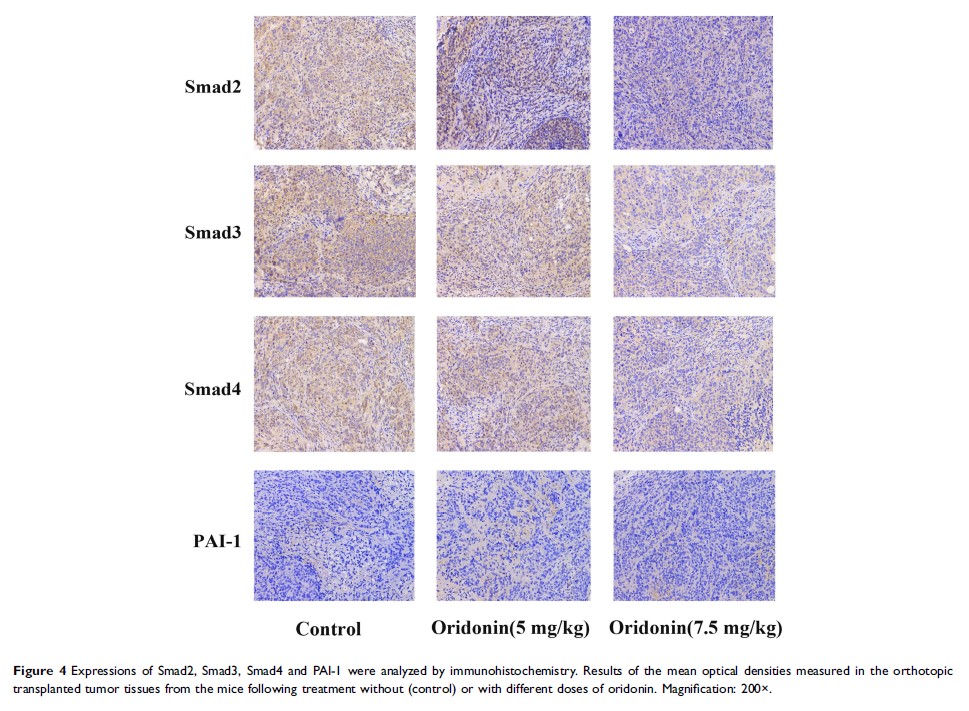
Results: Oridonin inhibited the proliferation of colon cancer LOVO cells in a concentration and time dependent manner. In addition, oridonin reduced the levels of Smad2, Smad3, Smad4, PAI-1 and the phosphorylation of Smad2 and Smad3 induced by TGF-β1 in vitro. Subsequently, we established an orthotopically implanted tumor model in nude mice and found that oridonin treatment significantly suppressed tumor growth, and which was accompanied by the down-regulation of Smad2, Smad3, Smad4, PAI-1 and p-Smad2, p-Smad3 expression levels.
Conclusion: Our present study demonstrated that the growth inhibition of colon cancer by oridonin could be partially mediated through discontinuing TGF-β1/Smads-PAI-1 signaling pathway, suggesting it as a promising agent in treating colorectal cancer.
Keywords: oridonin, colon cancer, TGF-β/Smads pathway, PAI-1