108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

硼替佐米通过不同给药方法治疗新诊断的多发性骨髓瘤的疗效和安全性分析
Authors Zhang HM, Liu XY, Liu YZ, Liu LN, Lin QD, Song YP, Fang BJ
Received 11 June 2019
Accepted for publication 22 July 2019
Published 10 September 2019 Volume 2019:11 Pages 8295—8302
DOI https://doi.org/10.2147/CMAR.S218979
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev Srivastava
Background: This study aims to compare the efficacy and adverse reactions of bortezomib for treating newly diagnosed multiple myeloma (MM) through two different administration methods: intravenous (IV) injection and subcutaneous (SC) injection.
Methods: A retrospective analysis was performed in 205 patients with newly diagnosed MM, who were treated by the Department of Hematopathology, Henan Cancer Hospital, from June 2009 to December 2017. These patients were divided into two groups according to the treatment methods: IV injection group, IV injection of bortezomib; SC injection group, SC injection of bortezomib.
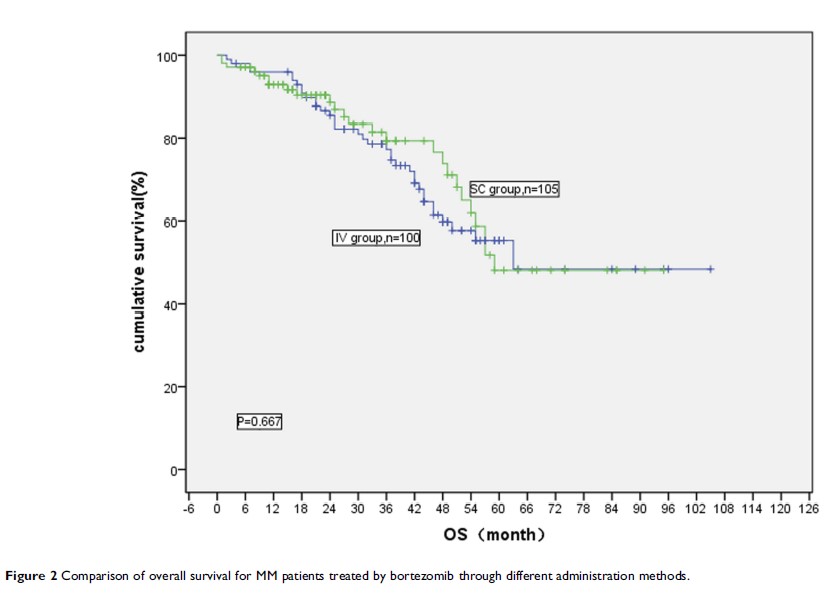
Results: After the first course of treatment, the effect of very good partial remission (VGPR) or above (≥VGPR) in the IV injection group (IV group) and SC injection group (SC group) was 31.0% and 14.3%, respectively (P =0.004), while the overall response rate (ORR) was 72.0% and 49.5%, respectively (P =0.001). From the 2nd course to the 6th course of treatment, the ORR was not statistically different between these two groups. No significant difference was found in median progression-free survival (37 vs 45 months) and overall survival (63 vs 59 months). A lower frequency of adverse events, especially Grade 3 peripheral neuropathy, was observed in SC group compared with the IV group.
Conclusion: Compared with IV administration, SC bortezomib can provide a better balance between efficacy and toxicity.
Keywords: multiple myeloma, bortezomib, intravenous injection, subcutaneous injection