108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

反复静脉注射二氧化硅纳米颗粒可在体内通过 JAK2/STAT3 和 TGF-β/Smad3 通路诱导肺部炎症和胶原蛋白积聚
Authors Yu Y, Zhu T, Li Y, Jing L, Yang M, Li Y, Duan J, Sun Z
Received 20 March 2019
Accepted for publication 30 July 2019
Published 6 September 2019 Volume 2019:14 Pages 7237—7247
DOI https://doi.org/10.2147/IJN.S209458
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Ms Justinn Cochran
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
Background: The health hazards of silica nanoparticle (SiNP) are raising worldwide concern as SiNPs has become the second largest manufactured nanomaterial in global markets. However, insufficient data for the adverse health effects and safety evaluation of SiNPs are remaining a big question.
Purpose: We evaluated the effects and related mechanism of SiNPs on pulmonary inflammation and collagen production through repeated intravenous administration in mice in a 45-day observation period.
Methods: Morphological and ultrastructural change, ultradistribution of SiNPs in lungs were observed in ICR mice through intravenous administration. Oxidative damage, pro-inflammatory cytokines, hydroxyproline content, the marker of fibroblasts and epithelial-mesenchymal transition (EMT), and JAK2/STAT3 and TGF-β1/Smad3 signaling pathways were detected to explore the lung injuries and related mechanism.
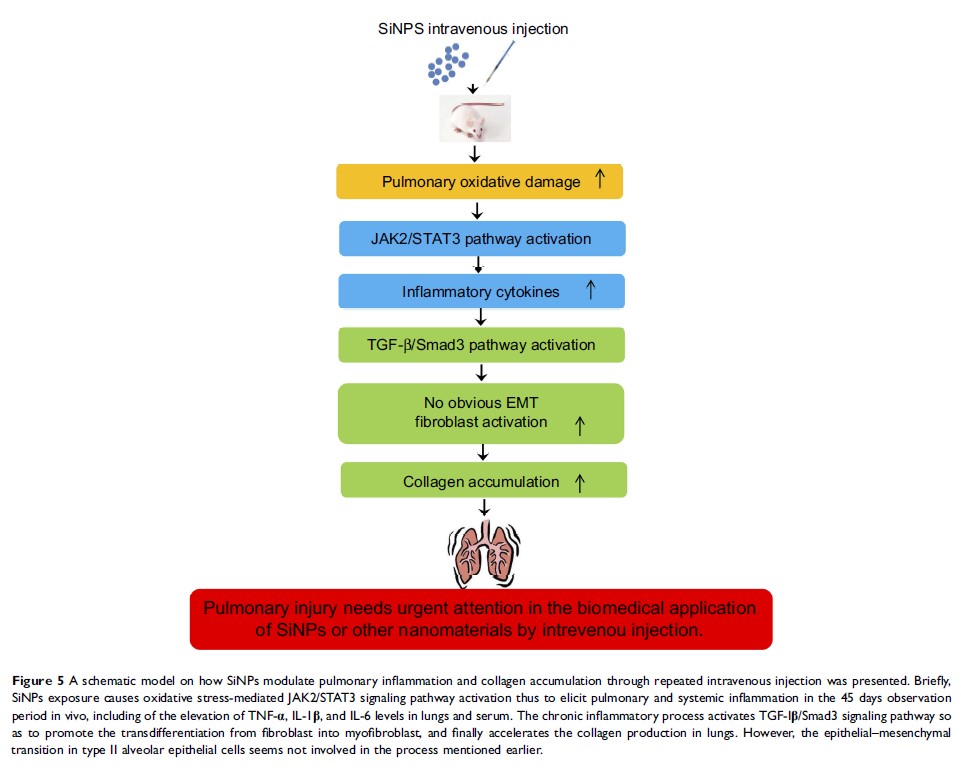
Results: The results showed repeated intravenous exposure of SiNPs increased the weight of lung tissues and destroyed pulmonary histomorphological structure. The increased MDA content, depletion of SOD and GSH-Px in lungs were observed in SiNP-treated mice. The protein expressions of JAK2/STAT3 pathway were upregulated in lungs, and the levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 in serum and lungs were also elevated in SiNPs treated group. The increased hydroxyproline content indicated collagen accumulation in lungs of SiNP-treated mice. Meanwhile, the protein expressions of the marker of myofibroblast (a-SMA), the regulators in connective tissue remodeling (CTGF), TGF-β, and p-Smad3 were all upregulated in lungs. In addition, we found intravenous administration of SiNPs-induced ultrastructural changes in type II alveolar epithelial cells but without downregulation of the protein expression of the key markers of epithelial cells (E-Cadherin).
Conclusion: Our results revealed that oxidative stress and inflammation contributed to the collagen accumulation through activation of JAK2/STAT3 and TGF-β/Smad3 pathways. It suggests that pulmonary aberrant inflammation and collagen accumulation induced by nanoparticles should be seriously considered for the safety application in diagnostics or therapeutics.
Keywords: Silica nanoparticles, repeated intravenous administration, inflammation, collagen accumulation, JAK2/STAT3 signaling pathway, TGF-β/Smad3 signaling pathway