108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

尼妥珠单抗联合同步放化疗治疗局部晚期宫颈癌的临床研究
Authors Chen W, Li T, Wang J, Liang L, Huang D, Yan G, Tian Y, Zhang X, Zhang W
Received 17 October 2018
Accepted for publication 11 May 2019
Published 3 September 2019 Volume 2019:11 Pages 8157—8165
DOI https://doi.org/10.2147/CMAR.S191134
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 3
Editor who approved publication: Dr Rituraj Purohit
Purpose: Nimotuzumab is a humanized monoclonal antibody that targets the epidermal growth factor receptor (EGFR) to inhibit tumor growth. Nimotuzumab has demonstrated desirable therapeutic activity in various types of tumors. However, the benefit of nimotuzumab for the treatment of cervical cancer is not entirely clear. The present study aimed to investigate the effects of nimotuzumab in the presence of CCRT in the first-line treatment of locally advanced cervical cancer (LACC).
Methods: The therapeutic efficacy and side effects of nimotuzumab combined with concurrent radiochemotherapy (CCRT) were retrospectively assessed in inoperable patients with LACC (stage IIb-IIIb) who were treated using CCRT with or without nimotuzumab.
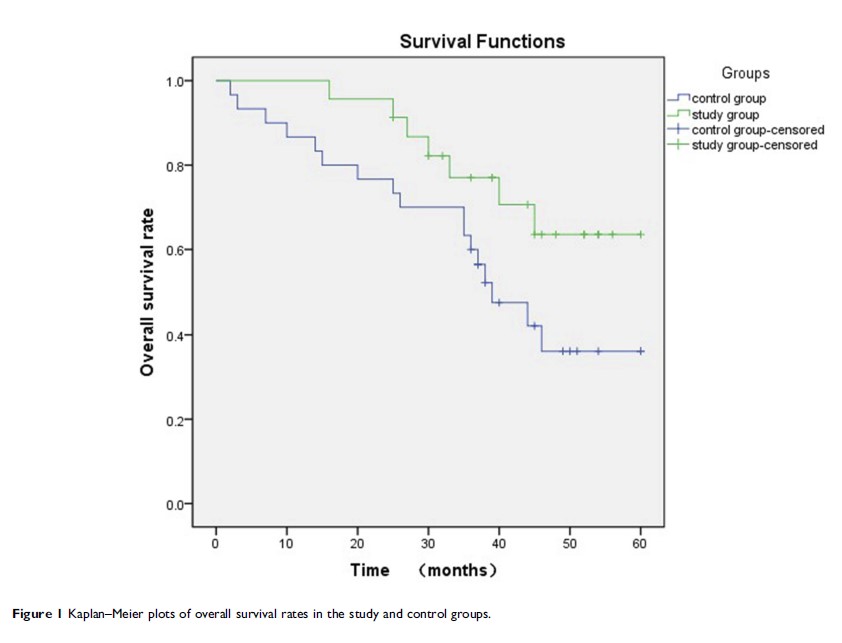
Results: The complete response rate of study group was significantly better than control group (78.3% vs 50%, P =0.035). The difference in median progression-free survival (PFS) in the two groups was statistically significan (not reach vs 27 months, P =0.037). Multivariate comparisons of prognostic factors in the two groups indicated that both the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) stage and combined nimotuzumab treatment affected PFS (P <0.05). Although generally tolerable, grade 3–4 toxicities including leukopenia (P =0.025) and hemoglobin (P =0.026) reduction were more frequent in the control group than those in the study group.
Conclusion: These data suggest that combining nimotuzumab with CCRT for the treatment of LACC resulted in extended PFS and higher complete remission rates, without an increased incidence of adverse events.
Keywords: EGFR monoclonal antibody, Nimotuzumab, cervical cancer, concurrent radiochemotherapy, PFS