108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Bombesin 功能化的超顺磁性氧化铁纳米粒子用于乳腺癌小鼠模型中的 MR/NIRFI双模
Authors Li L, Wu C, Pan L, Li X, Kuang A, Cai H, Tian R
Received 8 April 2019
Accepted for publication 6 July 2019
Published 21 August 2019 Volume 2019:14 Pages 6721—6732
DOI https://doi.org/10.2147/IJN.S211476
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Jiang Yang
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Background: The early and accurate detection afforded by imaging techniques significantly reduces mortality in cancer patients. However, it is still a great challenge to achieve satisfactory performance in tumor diagnosis using any single-modality imaging method. Magnetic resonance imaging (MRI) has excellent soft tissue contrast and high spatial resolution, but it suffers from low sensitivity. Fluorescence imaging has high sensitivity, but it is limited by penetration depth. Thus, the combination of the two modes could result in synergistic benefits. Here, we design and characterize a novel dual-modality MR/near-infrared fluorescence imaging (MR/NIRFI) nanomicelle and test its imaging properties in mouse models of breast cancer.
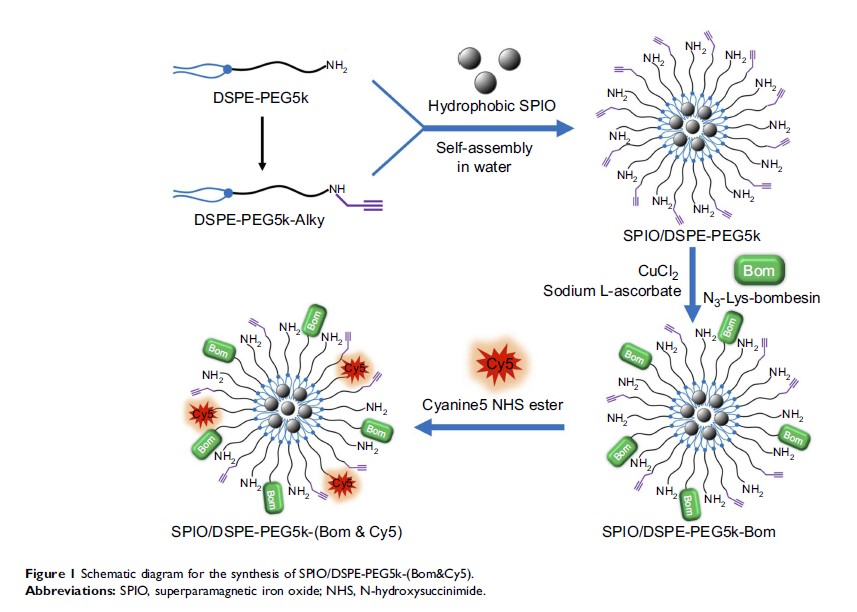
Methods: The nanomicelles were prepared by incorporating superparamagnetic iron oxide (SPIO) nanoparticles into 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-5000] micelles to which an NIRF dye and a tumor-targeted peptide (N3-Lys-bombesin, Bom) were conjugated. The nanomicelles were characterized for particle size, zeta potential and morphology. The transverse relaxivity, targeting specificity and imaging ability of the nanomicelles for MR/NIRFI were also examined.
Results: The fabricated nanomicelles displayed a well-defined spherical morphology with a mean diameter of 145±56 nm and a high transverse relaxivity (493.9 mM−1·s−1 , 3.0T). In MRI, the T2 signal reduction of tumors in the Bom-targeted group was 24.1±5.7% at 4 hrs postinjection, whereas only a 0.1±3.4% (P =0.003) decrease was observed in the nontargeted group. In NIRFI, the contrast increased gradually in the targeted group, and the tumor/muscle ratio increased from 3.7±0.3 at 1 hr to 4.7±0.1 at 2 hrs and to 6.4±0.2 at 4 hrs. No significant changes were observed in the nontargeted group at any time points.
Conclusion: Considering all our results, we conclude that these novel MR/NIRFI dual-modality nanomicelles could be promising contrast agents for cancer diagnosis.
Keywords: SPIO nanoparticles, magnetic resonance imaging, near-infrared fluorescence imaging, tumor diagnosis