108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

奥比妥珠单抗治疗慢性淋巴细胞白血病的临床应用
Authors Luan C, Chen B
Received 16 April 2019
Accepted for publication 14 July 2019
Published 19 August 2019 Volume 2019:13 Pages 2899—2909
DOI https://doi.org/10.2147/DDDT.S212500
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Melinda Thomas
Peer reviewer comments 3
Editor who approved publication: Professor Jianbo Sun
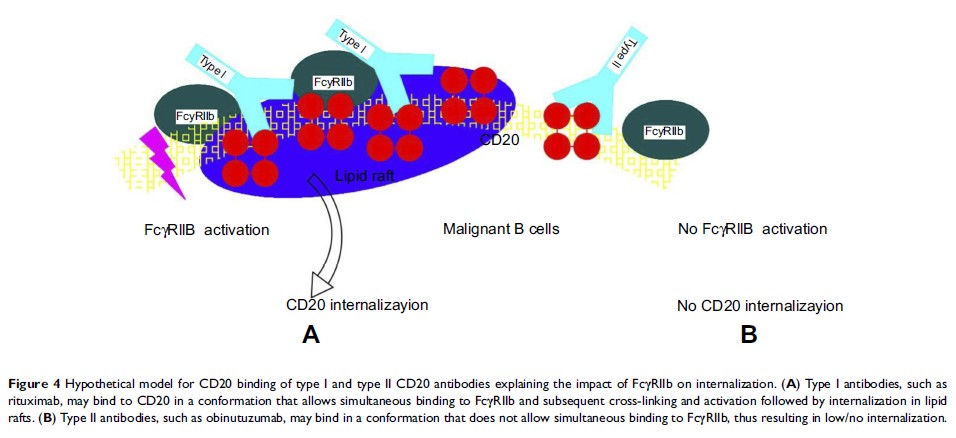
Abstract: Alkylators and nucleoside analogs were the main drugs for treatingchronic lymphoblastic leukemia (CLL), which have been replaced by monoclonal antibodies, such as rituximab in the past 10 years for refractory or relapsed CLL. The first-line immunochemotherapy regimen, rituximab combined with nucleoside analogs, significantly increased CLL patients’ first-reaction rate and improved progression-free survival. Despite the long-lasting remissions by the use of chemoimmunotherapy, most CLL patients will relapse eventually. The obinutuzumab (GA101), an updated CD20 antibody, that is thought to achieve a more durable response with unique molecular and functional characteristics. Obinutuzumab is a humanized, monoclonal type II CD20 antibody modified by glycoengineering. The glycoengineered Fc portion enhances the binding affinity to the FcγRIII receptor on immune effector cells, resulting in increased antibody-dependent cellular cytotoxicity and phagocytosis. In addition, the type II antibody binding characteristics of obinutuzumab to CD20 lead to an efficient induction of direct non-apoptotic cell death. This review summarizes the results of clinical studies using obinutuzumab and looks forward to its further application in treating CLL clinically.
Keywords: CD20 antibody, GA101, obinutuzumab, chronic lymphocytic leukemia