108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

第二代 EGFR 和 ErbB 酪氨酸激酶抑制剂作为非小细胞肺癌的一线治疗方法
Authors Wang S, Li J
Received 21 December 2018
Accepted for publication 13 May 2019
Published 15 August 2019 Volume 2019:12 Pages 6535—6548
DOI https://doi.org/10.2147/OTT.S198945
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Jyoti Bajaj
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
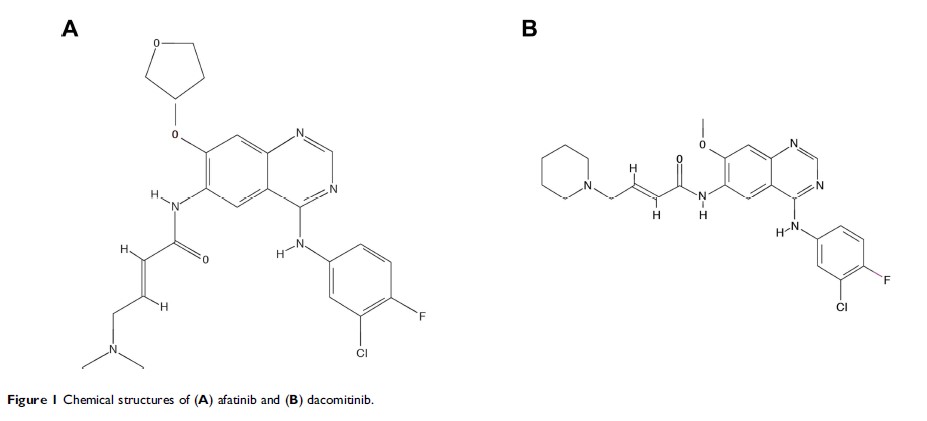
Abstract: The discovery that mutations in the EGFR gene are present in up to 50% of patients with lung adenocarcinoma, and the development of highly efficacious EGFR tyrosine kinase inhibitors (TKIs), has revolutionized the way this common malignancy is treated. Three generations of EGFR TKIs are now approved for use in EGFR mutation-positive non-small cell lung cancer (NSCLC); the first-generation agents erlotinib, gefitinib, and icotinib; the second-generation ErbB family blockers afatinib and dacomitinib; and most recently, osimertinib, a third-generation EGFR TKI. The second-generation agents have demonstrated impressive efficacy relative to both standard platinum-based chemotherapy and first-generation EGFR TKIs, significantly improving response and progression-free and overall survival. Data from real-world studies suggest that afatinib is as effective and well tolerated in routine clinical practice as it is in clinical studies and is effective in patients with certain uncommon EGFR mutations, patients with brain metastases, and older patients. Few real-world data are available for dacomitinib in the first-line setting. Afatinib and dacomitinib have similar safety profiles, with acne/skin dryzness, diarrhea, stomatitis, and paronychia the most common adverse events (AEs) reported in clinical and real-world studies. Numerous studies have shown that tolerability-guided dose reductions can help manage afatinib-related AEs without reducing efficacy. As the number of therapeutic options for advanced NSCLC increases, the optimal choice for first-line treatment will be determined by considering patient factors such as the presence of brain metastases, the type of EGFR mutation, tolerability, and subsequent therapy options for long-term treatment.
Keywords: afatinib, dacomitinib, epidermal growth factor receptor, non-small-cell lung cancer