108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

透明质酸修饰的介孔二氧化硅涂层超顺磁性 Fe3O4 纳米颗粒可用于靶向给药
Authors Fang Z, Li X, Xu Z, Du F, Wang W, Shi R, Gao D
Received 30 April 2019
Accepted for publication 6 July 2019
Published 30 July 2019 Volume 2019:14 Pages 5785—5797
DOI https://doi.org/10.2147/IJN.S213974
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Farooq Shiekh
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
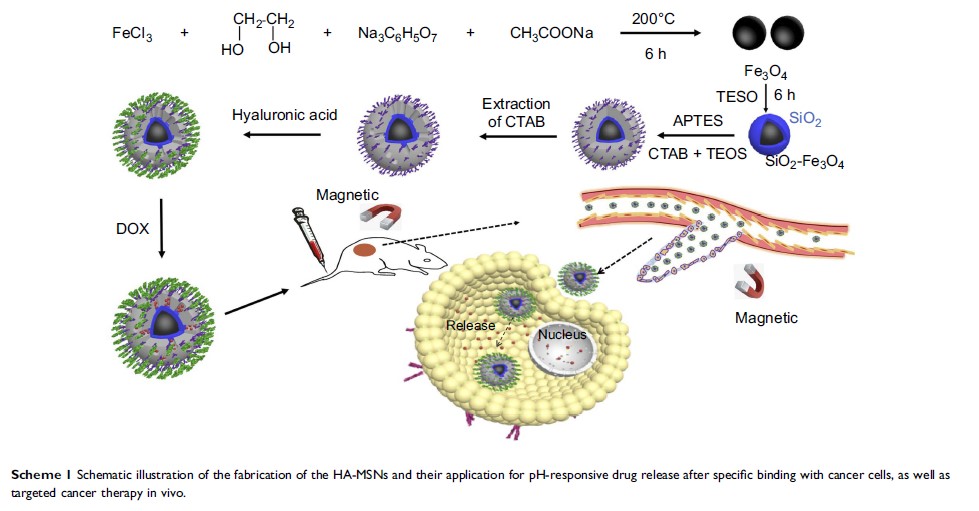
Introduction: The targeted delivery of anti-cancer drugs to tumor tissue has been recognized as a promising strategy to increase their therapeutic efficacy and reduce side effects. Mesoporous silica-coated superparamagnetic Fe3O4 nanoparticles (NH2-MSNs), a kind of nanocarrier, can passively enter tumor tissues to enhance the permeability and retention of drugs. However, NH2-MSNs do not specifically bind to cancer cells. This drawback encouraged us to develop a more efficient nanocarrier for cancer therapy.
Methods: Herein, we describe the development of an effective nanocarrier based on NH2-MSNs, which were modified with hyaluronic acid on their surface (HA-MSNs) and loaded with doxorubicin (DOX). We have successfully fabricated uniform spherical HA-MSNs nanocarriers. The targeting ability of this delivery system was evaluated through specific uptake by cells and IVIS imaging.
Results: DOX-HA-MSNs nanocarriers displayed more dramatic cytotoxic activity against 4T1 breast cancer cells compared to GES-1 gastric mucosa cells. In vivo results revealed that once DOX-HA-MSNs nanocarriers are exposed to an external magnetic field, they could be rapidly attracted to the magnet and effectively cross the cytoplasmic membrane via CD44 receptor-mediated transcytosis. This allows them to access the cancer cell cytoplasm and release DOX based on changes in the physiological environment. Both in vitro and in vivo results demonstrated that the HA-MSNs nanocarriers provided better therapeutic efficacy.
Conclusion: The HA-MSNs nanocarriers represent an effective new paradigm to treat cancers due to active targeting to the tumor cells. Moreover, the specific uptake by the tumor effectively protects normal tissues to reduce off-target side effects. The reported findings support further investigation of HA-MSNs for cancer therapy.
Keywords: target-delivery, HA-MSNs, nanocarrier, receptor-mediated, cancer therapy