109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
使用肿瘤基质渗透纳米载体的磁共振引导的区域基因传递策略用于胰腺癌治疗
Authors Wang Q, Li J, An S, Chen Y, Jiang C, Wang X
Published Date July 2015 Volume 2015:10 Pages 4479—4490
DOI http://dx.doi.org/10.2147/IJN.S84930
Received 18 March 2015, Accepted 4 May 2015, Published 13 July 2015
Background: Gene therapy is a very promising technology for treatment of pancreatic
ductal adenocarcinoma (PDAC). However, its application has been limited by the
abundant stromal response in the tumor microenvironment. The aim of this study
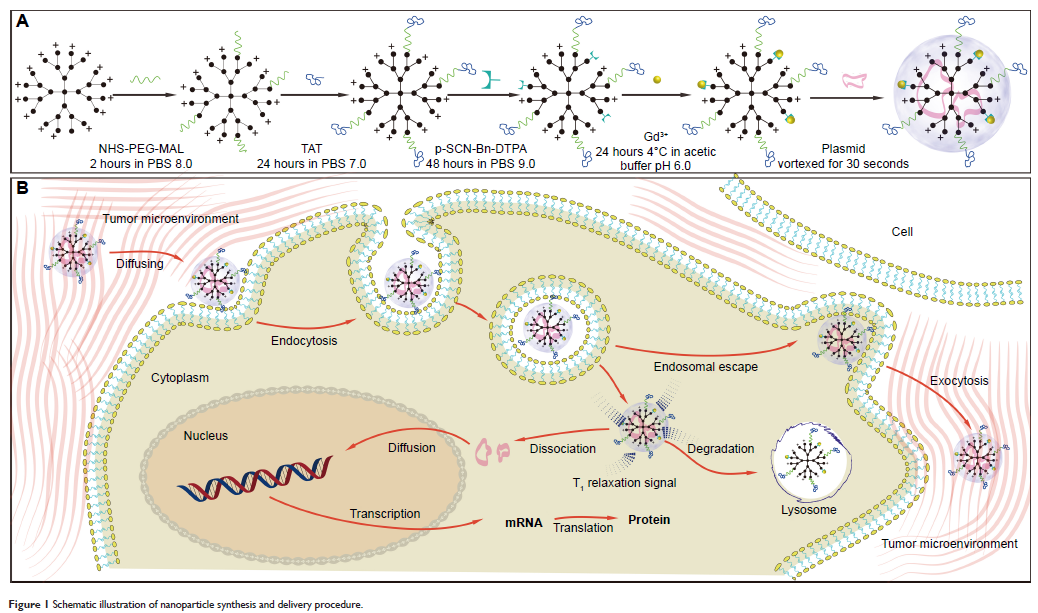
was to prepare a dendrimer-based gene-free loading vector with high
permeability in the tumor stroma and explore an imaging-guided local gene
delivery strategy for PDAC to promote the efficiency of targeted gene delivery.
Methods: The experimental protocol was approved by the animal ethics committee of Zhongshan Hospital, Fudan University. Third-generation dendrigraft poly-L-lysines was selected as the nanocarrier scaffold, which was modified by cell-penetrating peptides and gadolinium (Gd) chelates. DNA plasmids were loaded with these nanocarriers via electrostatic interaction. The cellular uptake and loaded gene expression were examined in MIA PaCa-2 cell lines in vitro. Permeability of the nanoparticles in the tumor stroma and transfected gene distribution in vivo were studied using a magnetic resonance imaging-guided delivery strategy in an orthotopic nude mouse model of PDAC.
Results: The nanocarriers were synthesized with a dendrigraft poly-L-lysine to polyethylene glycol to DTPA ratio of 1:3.4:8.3 and a mean diameter of 110.9±7.7 nm. The luciferases were strictly expressed in the tumor, and the luminescence intensity in mice treated by Gd-DPT/plasmid luciferase (1.04×104±9.75×102 p/s/cm2/sr) was significantly (P <0.05) higher than in those treated with Gd-DTPA (9.56×102±6.15×10 p/s/cm2/sr) and Gd-DP (5.75×103± 7.45×102 p/s/cm2/sr). Permeability of the nanoparticles modified by cell-penetrating peptides was superior to that of the unmodified counterpart, demonstrating the improved capability of nanoparticles for diffusion in tumor stroma on magnetic resonance imaging.
Conclusion: This study demonstrated that an image-guided gene delivery system with a stroma-permeable gene vector could be a potential clinically translatable gene therapy strategy for PDAC.
Keywords: molecular imaging, magnetic resonance imaging, interventional, pancreatic cancer, genetic therapy, cell-penetrating peptides
Methods: The experimental protocol was approved by the animal ethics committee of Zhongshan Hospital, Fudan University. Third-generation dendrigraft poly-L-lysines was selected as the nanocarrier scaffold, which was modified by cell-penetrating peptides and gadolinium (Gd) chelates. DNA plasmids were loaded with these nanocarriers via electrostatic interaction. The cellular uptake and loaded gene expression were examined in MIA PaCa-2 cell lines in vitro. Permeability of the nanoparticles in the tumor stroma and transfected gene distribution in vivo were studied using a magnetic resonance imaging-guided delivery strategy in an orthotopic nude mouse model of PDAC.
Results: The nanocarriers were synthesized with a dendrigraft poly-L-lysine to polyethylene glycol to DTPA ratio of 1:3.4:8.3 and a mean diameter of 110.9±7.7 nm. The luciferases were strictly expressed in the tumor, and the luminescence intensity in mice treated by Gd-DPT/plasmid luciferase (1.04×104±9.75×102 p/s/cm2/sr) was significantly (P <0.05) higher than in those treated with Gd-DTPA (9.56×102±6.15×10 p/s/cm2/sr) and Gd-DP (5.75×103± 7.45×102 p/s/cm2/sr). Permeability of the nanoparticles modified by cell-penetrating peptides was superior to that of the unmodified counterpart, demonstrating the improved capability of nanoparticles for diffusion in tumor stroma on magnetic resonance imaging.
Conclusion: This study demonstrated that an image-guided gene delivery system with a stroma-permeable gene vector could be a potential clinically translatable gene therapy strategy for PDAC.
Keywords: molecular imaging, magnetic resonance imaging, interventional, pancreatic cancer, genetic therapy, cell-penetrating peptides