108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

西妥昔单抗联合治疗晚期鼻咽癌患者:荟萃分析
Authors Shen J, Sun C, Zhou M, Zhang Z
Received 1 November 2018
Accepted for publication 1 February 2019
Published 3 April 2019 Volume 2019:12 Pages 2477—2494
DOI https://doi.org/10.2147/OTT.S193039
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev Srivastava
Purpose: Cetuximab,
an anti-epidermal growth factor receptor monoclonal antibody, carries the
potential for combination treatment against nasopharyngeal carcinoma (NPC). We
conducted a meta-analysis to assess the possible benefits and safety between
the combination treatment with cetuximab and conventional treatment in NPC
patients. Skin toxicity (ST) associated with additional cetuximab was evaluated
as well.
Methods: We
performed a systematic search (PubMed, Embase, Cochrane library, China National
Knowledge Infrastructure, and WanFang Data) for studies comparing combination
treatment with cetuximab versus conventional treatment in NPC patients. The
selected studies included completely or partly reported clinical outcomes
including survivals, complete and partial responses, and adverse reactions
(ST). The pooled HR, relative risk (RR), and respective 95% CI were estimated
by using fixed effects model or random effects model.
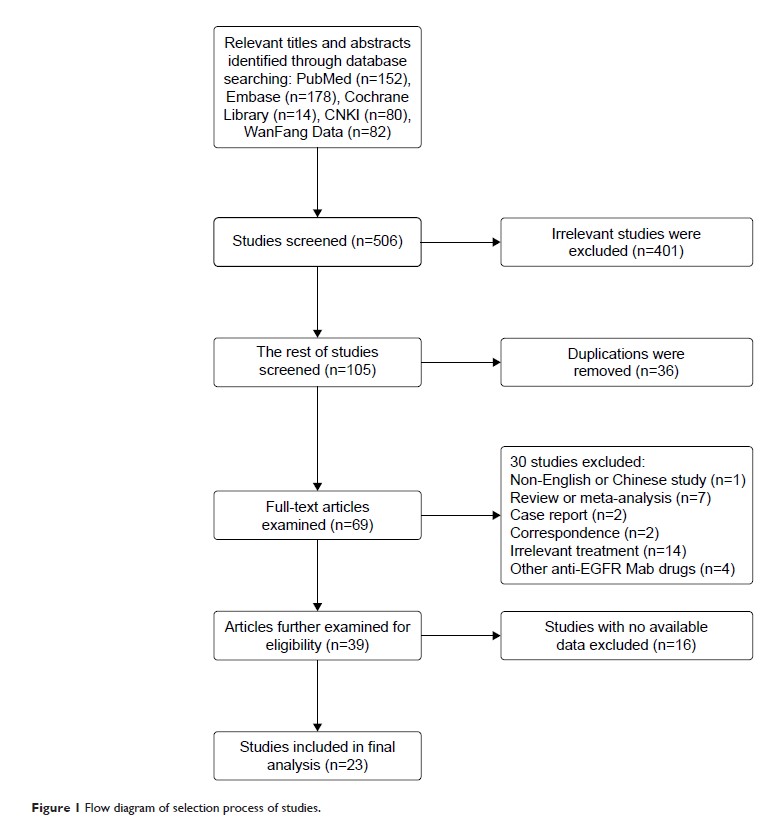
Results: A total
of 23 relevant studies with available data were included in the final analysis.
According to the pooled data, combination treatment with cetuximab showed
improved efficacy on increased objective response rate (studies with cetuximab
treatment: RR: 1.39, 95% CI: 1.29–1.50; concurrent chemoradiotherapy with or
without cetuximab: RR: 1.39, 95% CI: 1.25–1.54) and prolonged survival (studies
with cetuximab treatment: the pooled HR for OS was 0.70, 95% CI: 0.55–0.89;
concurrent chemoradiotherapy with or without cetuximab: the pooled HR for OS
was 0.64, 95% CI: 0.49–0.84) compared with conventional treatment. Moreover,
the improved efficacy was invariably accompanied by an increased occurrence of
ST (studies with cetuximab treatment: RR: 2.46, 95% CI: 1.81–3.34; concurrent
chemoradiotherapy with or without cetuximab: RR: 1.84, 95% CI: 1.02–3.31). However,
the majority of adverse reactions exhibited similar occurrence rates between
the different treatments.
Conclusion: Patients
with NPC receiving additional cetuximab treatment can benefit more from this
systemic comprehensive therapy, while the efficiency of conventional treatment
for NPC is limited. ST associated with cetuximab may be used as a potential
on-treatment marker to guide treatment with cetuximab against NPC.
Keywords: nasopharyngeal
carcinoma, cetuximab, combination treatment, clinical outcomes