108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

对代偿性乙型肝炎病毒相关性肝硬化进行恩替卡韦单药治疗与拉米夫定和阿德福韦的新联合治疗的对比:一项现实世界的前瞻性多中心队列研究
Authors Wu X, Zhou J, Xie W, Ding H, Ou X, Chen G, Ma A, Xu X, Ma H, Xu Y, Liu X, Meng T, Wang L, Sun Y, Wang B, Kong Y, Ma H, You H, Jia J
Received 24 August 2018
Accepted for publication 11 February 2019
Published 1 April 2019 Volume 2019:12 Pages 745—757
DOI https://doi.org/10.2147/IDR.S185120
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Background: De novo combination of lamivudine (Lam)
and adefovir (Adv) was not rarely used in clinical practice. However, head-to-head
comparisons of entecavir (Etv) monotherapy with this combination in hepatitis B
virus (HBV)-related compensated cirrhosis patients are unavailable. This study
aimed to compare the efficacy and safety of Etv monotherapy with combination
therapy in patients with HBV-related compensated liver cirrhosis.
Methods: Treatment-naïve
patients with HBV-related compensated liver cirrhosis were recruited to receive
either Etv monotherapy or a de novo combination of Lam and Adv. Data were
collected at baseline and every 6 months thereafter.
Results: A
total of 578 patients (485 in Etv group, 93 in combination group) were
included. Baseline characteristics were comparable between the two groups. At
the end of 1, 2, and 3 years, HBV DNA was undetectable in 82.7%, 96.2%, and
94.3% of patients in the Etv group and 88.9%, 81.7%, and 84.6% in the
combination group, respectively (all P >0.05). The cumulative virological
breakthrough rate at 1, 2, and 3 years was 2.7%, 6.7%, and 9.8% in the Etv
group and 2.9%, 13.3%, and 32.2% in the combination group, respectively (P =0.003). After
propensity-score adjustment for age, sex, and baseline HBeAg, ALT, and total
bilirubin, virological breakthrough was higher in the de novo combination of
Lam and Adv (HR 2.83, 95% CI 1.37–5.86; P <0.01). The cumulative rate of liver-related
events, including decompensation and hepatocellular carcinoma, at 1, 2, and 3
years was 2.9%, 4.2%, and 6.1% in the Etv group and 2.2%, 2.2%, and 6.7% in
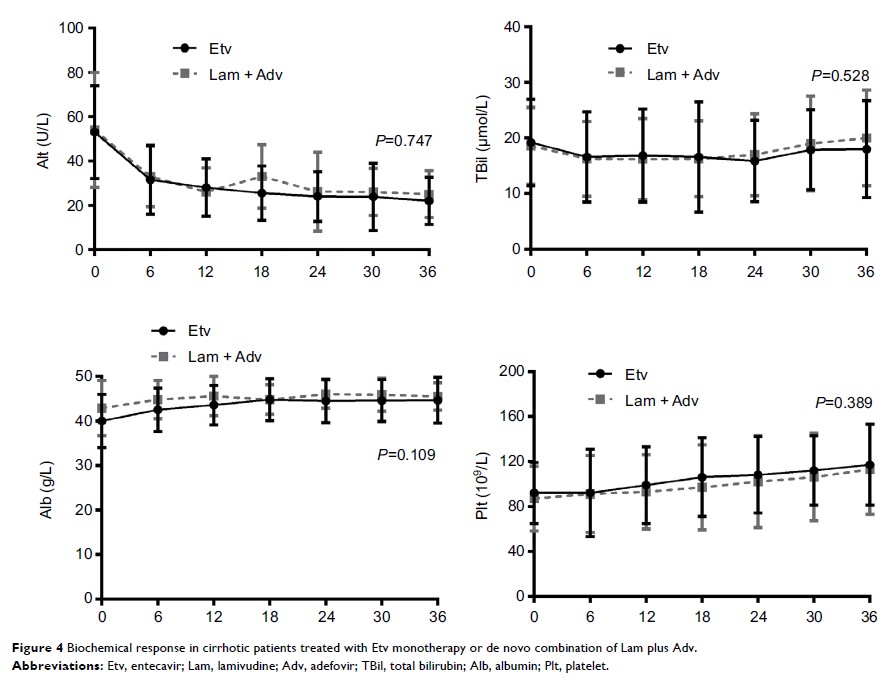
combination group, respectively (P =0.83). Biochemical response and serological response
were similar between the groups.
Conclusion: Etv
treatment had less virological breakthrough and potentially higher HBV-DNA
suppression than de novo combination of Lam and Adv during 3 years in
treatment-naïve HBV-related compensated liver cirrhosis.
Keywords: entecavir,
de novo combination, lamivudine, adefovir, compensated HBV-related cirrhosis,
real-world, virological breakthrough