9 7 8 1 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.3 Breast Cancer (Dove Med Press)
- 3.4 Clin Epidemiol
- 2.5 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.5 Clin Interv Aging
- 4.7 Drug Des Dev Ther
- 2.7 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.5 Int J Women's Health
- 2.5 Neuropsych Dis Treat
- 2.7 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.3 Ther Clin Risk Manag
- 2.5 J Pain Res
- 2.8 Diabet Metab Synd Ob
- 2.8 Psychol Res Behav Ma
- 3.0 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.7 Risk Manag Healthc Policy
- 4.2 J Inflamm Res
- 2.1 Int J Gen Med
- 4.2 J Hepatocell Carcinoma
- 3.7 J Asthma Allergy
- 1.9 Clin Cosmet Investig Dermatol
- 2.7 J Multidiscip Healthc

抑制人类胶质母细胞瘤细胞侵袭与 PION@E6 介导的自噬过程有关
Authors Ren Z, Liang J, Zhang P, Chen J, Wen J
Received 2 January 2019
Accepted for publication 13 February 2019
Published 29 March 2019 Volume 2019:11 Pages 2643—2652
DOI https://doi.org/10.2147/CMAR.S200151
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Background: Glioblastoma
(GBM) is the most severe brain cancer due to its ability to invade surrounding
brain tissue. Iron oxide nanoparticles (ION) could effectively induce a
decrease of cell migration/invasion. Also IONs could generate ROS stress which
induces autophagy elevation. Autophagy is associated with both
anti-tumorigenesis and protumorigenesis.
Objective: To
explore the effect of PEGylated IONs (PION@E6) on the GBM cell invasion and its
mechanism based on autophagy.
Materials and methods: PION@E6
were prepared and characterized according to our previous study. After
incubation of U251 cells with PION@E6, cellular uptake of PION@E6 and cell
viability were tested by Prussian blue staining and Cell Counting Kit-8,
respectively. The migration and invasive capability was assessed by transwell
cell migration and invasion assay. Expressions of autophagy biomarkers were
detected by Western blotting. Intracellular ROS level was determined using
2′–7′-dichlorodihydrofluorescein diacetate.
Results: Average
hydrate particle size and zeta potential of PION@E6 were 37.86±12.90 nm and
–23.8 mV, respectively, and uniformly distributed nanoparticles with an average
diameter of 10 nm were observed by TEM. Chlorin e6 successfully incorporated
onto PION@E6 was demonstrated by ultraviolet and visible absorption
spectrophotometry, and PION@E6 owning excellent water solubility and stability
were showed by Colloid stability test. PION@E6 were successfully taken up by
U251 cells with Prussian blue staining, and they showed in vitro cytotoxicity
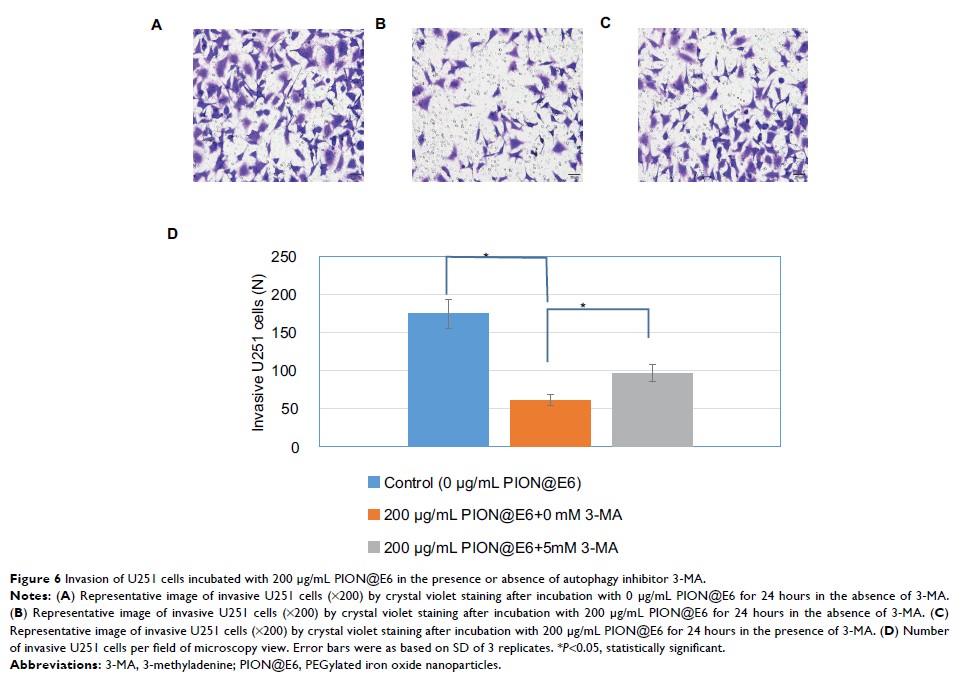
to glioma cells after long incubation of 72 hours. Migration/invasion of cells
was significantly inhibited by PION@E6, which could be counteracted by
pretreatment with 3-MA. Additionally, the expression of beclin-1, IC3I, and IC3II
proteins was higher, whereas that of p62 protein was lower. Moreover, a dose
dependent intracellular ROS generation of PION@E6 was detected.
Conclusion: Invasiveness
of human GBM cells involves the PION@E6-mediated autophagy process, which may
be related to the intracellular ROS induced by PION@E6.
Keywords: iron
oxide nanoparticle, glioblastoma, invasiveness, autophagy