9 7 8 1 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.3 Breast Cancer (Dove Med Press)
- 3.4 Clin Epidemiol
- 2.5 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.5 Clin Interv Aging
- 4.7 Drug Des Dev Ther
- 2.7 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.5 Int J Women's Health
- 2.5 Neuropsych Dis Treat
- 2.7 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.3 Ther Clin Risk Manag
- 2.5 J Pain Res
- 2.8 Diabet Metab Synd Ob
- 2.8 Psychol Res Behav Ma
- 3.0 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.7 Risk Manag Healthc Policy
- 4.2 J Inflamm Res
- 2.1 Int J Gen Med
- 4.2 J Hepatocell Carcinoma
- 3.7 J Asthma Allergy
- 1.9 Clin Cosmet Investig Dermatol
- 2.7 J Multidiscip Healthc

CHOP 与 CHOPE 治疗外周 T 细胞淋巴瘤的比较:荟萃分析
Authors Deng S, Lin S, Shen J, Zeng Y
Received 5 October 2018
Accepted for publication 19 December 2018
Published 28 March 2019 Volume 2019:12 Pages 2335—2342
DOI https://doi.org/10.2147/OTT.S189825
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Manfred Beleut
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Objective: To
compare cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and
CHOP plus etoposide (CHOPE) with regard to outcomes including efficacy and
safety for patients with peripheral T-cell lymphoma (PTCL).
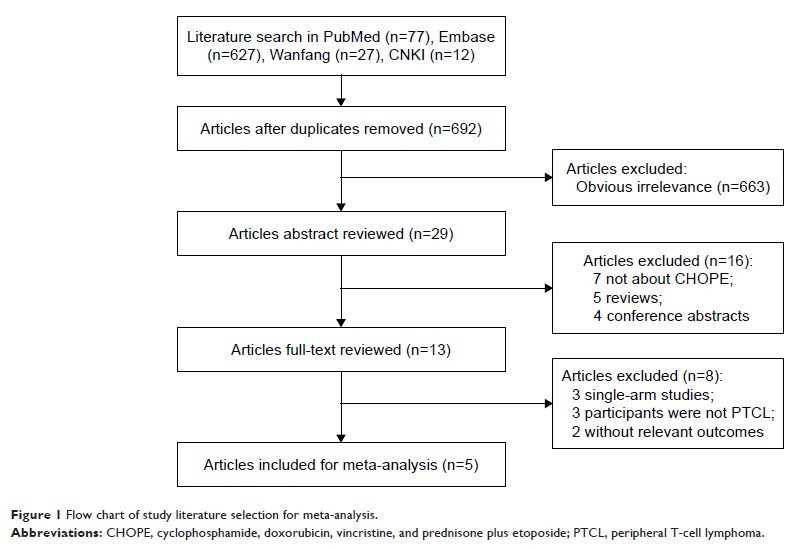
Methods: Relevant
literature was searched using PubMed, Embase, Wanfang, and CNKI for eligible
trials comparing CHOP with CHOPE for treatment of PTCL. The following outcomes
of PTCL patients were considered: complete response (CR), partial response
(PR), overall response rate (ORR), and adverse events (AEs; grade ≥3).
Risk ratios (RRs) were appropriately derived from fixed-effects or
random-effects models.
Results: A total
of five prospective or retrospective articles with 1,560 patients were elected
for the meta-analysis. There were no significant differences in CR (RR =1.11,
95% CI: 0.73–1.67, P =0.632), PR (RR =1.40, 95% CI: 0.52–3.76, P =0.504), and ORR
(RR =1.25, 95% CI: 0.93–1.69, P =0.146) between the CHOP and CHOPE groups. However,
AEs including anemia (RR =1.69, 95% CI: 1.33–2.16, P <0.001) and
thrombocytopenia (RR =1.43, 95% CI: 1.15–1.77, P =0.001) were
significantly increased in CHOPE group compared to that in CHOP group.
Conclusion: Meta-analysis
suggested that there were no differences in therapeutic effect for patients
with PTCL between CHOP and CHOPE groups with regards to CR, PR, and ORR,
whereas the CHOPE group had significantly increased AEs (anemia and
thrombocytopenia) compared to CHOP group.
Keywords: peripheral
T-cell lymphoma, complete response, partial response, overall response rate,
adverse events