9 7 8 1 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.3 Breast Cancer (Dove Med Press)
- 3.4 Clin Epidemiol
- 2.5 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.5 Clin Interv Aging
- 4.7 Drug Des Dev Ther
- 2.7 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.5 Int J Women's Health
- 2.5 Neuropsych Dis Treat
- 2.7 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.3 Ther Clin Risk Manag
- 2.5 J Pain Res
- 2.8 Diabet Metab Synd Ob
- 2.8 Psychol Res Behav Ma
- 3.0 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.7 Risk Manag Healthc Policy
- 4.2 J Inflamm Res
- 2.1 Int J Gen Med
- 4.2 J Hepatocell Carcinoma
- 3.7 J Asthma Allergy
- 1.9 Clin Cosmet Investig Dermatol
- 2.7 J Multidiscip Healthc

JAM3 作为一种新型肿瘤抑制因子起作用,并在结直肠癌中被 DNA 甲基化失活
Authors Zhou D, Tang W, Zhang Y, An HX
Received 6 October 2018
Accepted for publication 12 February 2019
Published 27 March 2019 Volume 2019:11 Pages 2457—2470
DOI https://doi.org/10.2147/CMAR.S189937
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Purpose: JAM3, an
adhesion and transmigration regulatory element, is abundantly expressed in
intestinal epithelial cells. However, its expression and function in colorectal
cancer (CRC) remain unknown. In this study, we explored its epigenetic
mechanism and biological role in CRC.
Patients and methods: Bioinformatics
analysis was used to analyze the expression and methylation level of JAM3 in
CRC. Methylation and expression status of JAM3 were then validated by
quantitative methylation-specific PCR (qMSP) and quantitative PCR in tissues,
plasma samples, and cell lines. Flow cytometry, Western blot, transwell, siRNA,
colony formation, and transfection were used to evaluate the biological
function of JAM3.
Results: We
initially found that JAM3 was frequently methylated and downregulated in CRC
based on bioinformatics tools. qMSP validation showed that the methylation
levels of JAM3 were increased in 75% (18/24) of CRC tissues, 61% (11/18) plasma
samples, and all four CRC cell lines and were significantly associated with
tumor stage in CRC tissues. Moreover, JAM3 was downregulated in primary CRC
tissues, plasma samples, and CRC cell lines as compared with that in
nonmalignant controls, although its expression could be recovered after
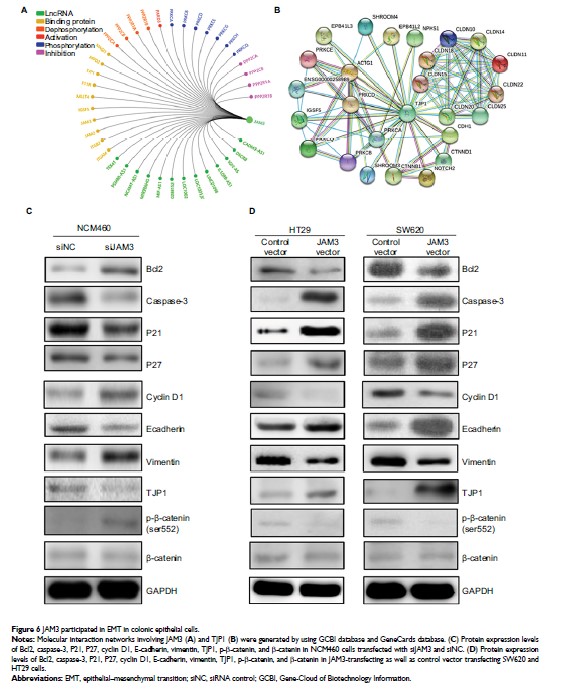
demethylation treatment. Restoration of JAM3 repressed CRC cell viability,
colony formation, and migration. In addition, siRNA-mediated depletion of JAM3
in NCM460 cells improved the clonogenicity and migration capability, whereas it
suppressed cell apoptosis and cell-cycle arrest. These functional effects were
accompanied with alterations of several epithelial cell markers, including
E-cadherin, vimentin, phosphor-β-catenin (ser552), and TJP1, which were
responsible for epithelial–mesenchymal transition.
Conclusion: The
findings indicated that JAM3 may be a novel tumor suppressor gene with
epigenetic reduction in CRC and can be used as a potential noninvasive
biomarker for CRC diagnosis.
Keywords: epigenetics,
EMT, migration, metastasis