9 7 8 1 6
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.3 Breast Cancer (Dove Med Press)
- 3.4 Clin Epidemiol
- 2.5 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.5 Clin Interv Aging
- 4.7 Drug Des Dev Ther
- 2.7 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.5 Int J Women's Health
- 2.5 Neuropsych Dis Treat
- 2.7 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.3 Ther Clin Risk Manag
- 2.5 J Pain Res
- 2.8 Diabet Metab Synd Ob
- 2.8 Psychol Res Behav Ma
- 3.0 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.7 Risk Manag Healthc Policy
- 4.2 J Inflamm Res
- 2.1 Int J Gen Med
- 4.2 J Hepatocell Carcinoma
- 3.7 J Asthma Allergy
- 1.9 Clin Cosmet Investig Dermatol
- 2.7 J Multidiscip Healthc

非特异性免疫球蛋白 G 在预防和治疗小鼠癌症方面的有效性
Authors Xu Q, Zhang Z, Chen Z, Zhang B, Zhao C, Zhang Y, Zhao C, Deng X, Zhou Y, Wu Y, Gu J
Received 20 September 2018
Accepted for publication 24 January 2019
Published 7 March 2019 Volume 2019:11 Pages 2073—2085
DOI https://doi.org/10.2147/CMAR.S188172
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Background: Previous
accidental findings showed that administration of immunoglobulin G (IgG) in
treating autoimmune diseases was able to inhibit cancers that happened to grow
in these patients. However, such treatment has not been used to treat cancer
patients clinically. The mechanism and optimal dosages of this treatment have
not been established. Subsequent animal experiments confirmed this effect, but
all previous studies in animal models used human IgG which was heterogeneous to
the animal hosts and therefore could adversely interfere with the results.
Materials and methods: We tested
different dosages of mouse IgG in treating and preventing three syngeneic
cancer types (melanoma, colon cancer, and breast cancer) in three immune potent
mouse models. The expression of Ki67, CD34, VEGF, MMPs, and cytokines in tumor
tissues were examined with immunohistochemistry or quantitative real-time PCR
to evaluate tumor proliferation, vascularization, metastasis, and
proinflammatory response in the tumor microenvironment.
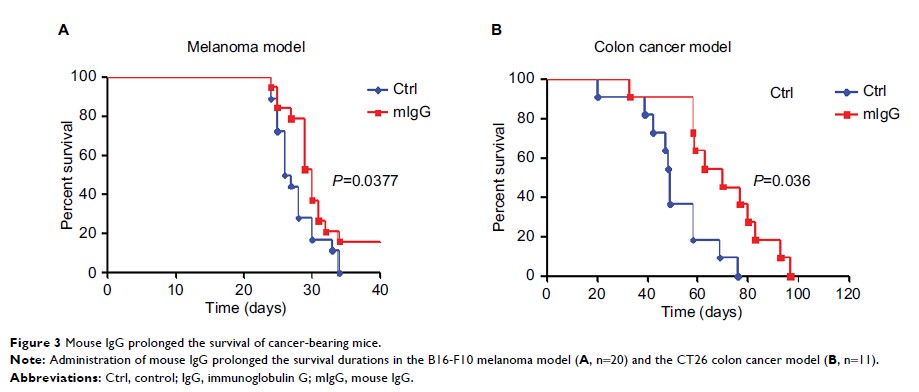
Results: We found
that low-dose IgG could effectively inhibit cancer progression, regulate tumor
vessel normalization, and prolong survival. Administration of IgG before cancer
cell inoculation could also prevent the development of cancer. In addition, IgG
caused changes in a number of cytokines and skewed macrophage polarization
toward M1-like phenotype, characterized by proinflammatory activity and
inhibition of proliferation of cancer cells.
Conclusion: Our
findings suggest that nonspecific IgG at low dosages could be a promising
candidate for cancer prevention and treatment.
Keywords: IVIg,
cancer therapy, macrophages, mouse model, immunotherapy