108899
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

RRAD 通过下调肝细胞癌中的 ACTG1 来抑制 Warburg 效应
Authors Yan Y, Xu H, Zhang L, Zhou X, Qian X, Zhou J, Huang Y, Ge W, Wang W
Received 11 December 2018
Accepted for publication 17 January 2019
Published 28 February 2019 Volume 2019:12 Pages 1691—1703
DOI https://doi.org/10.2147/OTT.S197844
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Purpose: Hepatocellular
carcinoma (HCC) is a common malignancy with poor prognosis and limited
therapeutic options. Ras-related associated with diabetes (RRAD) belongs to the
subfamily of Ras-related GTPases and is associated with several types of
cancer, including HCC, although the mechanisms involving RRAD in HCC remains
unknown.
Patients and methods: We aimed
to elucidate the role of RRAD and whether it affects glucose metabolism in HCC
by immunohistochemically examining tissue samples from HCC patients and
assessing the effect of RRAD overexpression and knockdown on the glucose
metabolism, proliferation, cell cycle, and apoptosis of HCC cell lines SK-Hep-1
and Huh7, as well as on tumor progression in vivo.
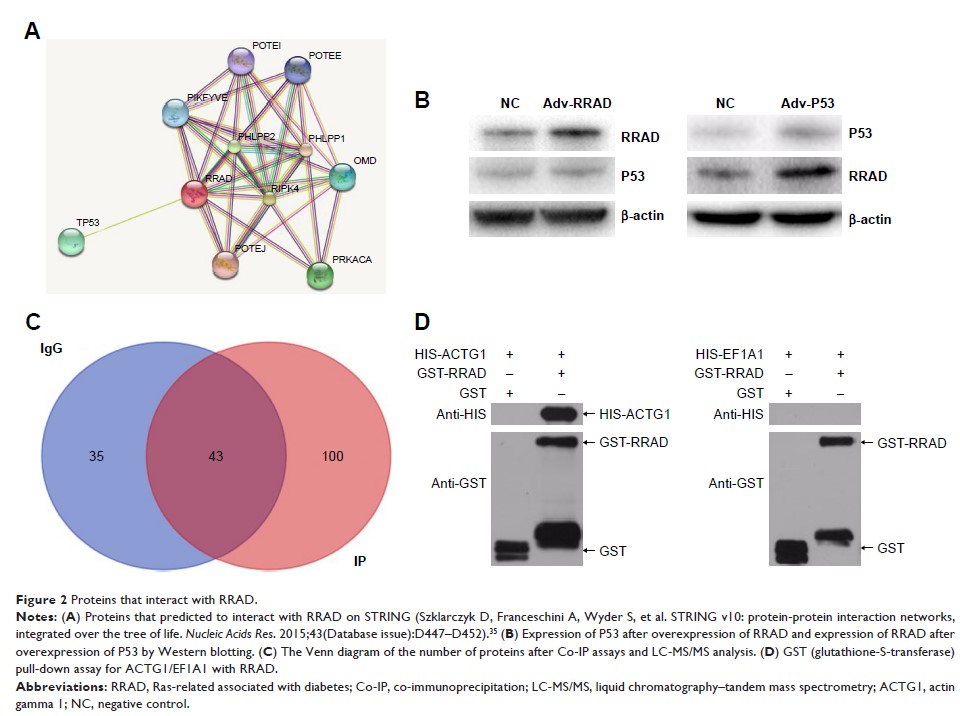
Results: We
demonstrated that RRAD binds to actin gamma 1 (ACTG1). RRAD suppressed aerobic
glycolysis in HCC by downregulating ACTG1. On the other hand, ACTG1 promoted
HCC proliferation by regulating the cell cycle via downregulation of cyclins
and cyclin-dependent kinases and inhibited apoptosis through the mitochondrial
apoptosis pathway in vitro. In addition, RRAD retarded tumor growth by
downregulating ACTG1 in vivo. ACTG1 was overexpressed in HCC tissues compared
with adjacent normal tissues, whereas the expression of RRAD was low in tumor
tissues. Low RRAD levels were significantly correlated with large tumor size
and advanced tumor stage; high ACTG1 levels were significantly correlated with
advanced tumor stage. Furthermore, Kaplan–Meier survival curves showed that HCC
patients with high RRAD and low ACTG1 expression may have a better prognosis.
Conclusion: We have
shown that RRAD exhibits a tumor-suppressing role in HCC by downregulating
glucose metabolism and ACTG1 expression, thus lowering cell proliferation,
arresting the cell cycle, and increasing apoptosis. These findings indicate
that ACTG1 may act as a downstream effector of RRAD and open a new avenue for
potential HCC treatment.
Keywords: hepatocellular
carcinoma, Ras-related associated with diabetes, actin gamma 1, the Warburg
effect, tumorigenicity