108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

为中国晚期鳞状细胞肺癌患者制定治疗方案:着重探讨阿法替尼
Authors Lu S
Received 21 September 2018
Accepted for publication 14 December 2018
Published 22 February 2019 Volume 2019:12 Pages 1521—1538
DOI https://doi.org/10.2147/OTT.S188296
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Abstract: Lung
cancer is the leading cause of cancer death in China, and approximately one
third of these cancers are squamous cell carcinoma (SqCC) of the lung. Ethnic
diversity and country-specific environmental factors can account for
interindividual variations in response to and tolerability of anticancer
therapies. Although several targeted therapies have recently been approved for
patients with relapsed/refractory SqCC of the lung, only afatinib, an
irreversible ErbB family blocker, has data of Chinese patients. In the Phase
III LUX-Lung 8 trial, afatinib demonstrated a significant clinical benefit vs
the reversible first-generation EGFR tyrosine kinase inhibitor erlotinib in
both the overall population and the Chinese subset, with a manageable safety
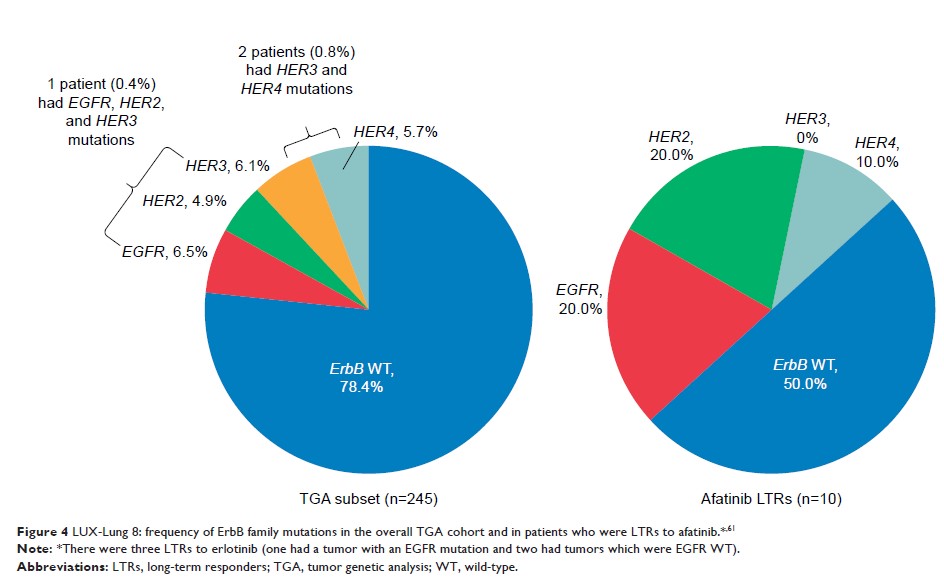
profile. Emerging biomarker data from LUX-Lung 8 suggest that patients with
ErbB mutations, especially ErbB2, and those classified as “good” in the
VeriStrat® proteomic test, may benefit from afatinib
treatment in particular, regardless of ethnicity, and may get a long-term
response. In conclusion, afatinib is a valid second-line option for Chinese
patients with SqCC of the lung, and specific biomarkers may help guide in
treatment decision-making. Ongoing studies will provide further guidance on
afatinib’s place in the treatment algorithm, alongside the other novel targeted
therapies.
Keywords: squamous
cell carcinoma, NSCLC, Chinese, afatinib, EGFR, ErbB, biomarker