108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

原钙粘蛋白 17 是肿瘤抑制剂,并且经常在鼻咽癌中甲基化
Authors He Y, Wang Z, Liu C, Gong Z, Li Y, Lu T, Hu G
Received 16 October 2018
Accepted for publication 13 January 2019
Published 18 February 2019 Volume 2019:11 Pages 1601—1613
DOI https://doi.org/10.2147/CMAR.S191102
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Purpose: Several PCDH genes
were shown to be downregulated or silenced in carcinomas and act as candidate
tumor suppressor genes. However, the functions of PCDH17 in
nasopharyngeal carcinoma (NPC) remain unclear. Here, we investigated the PCDH17 promoter
methylation status and its impact on the expression and functions of PCDH17 in NPC.
Patients and methods: To
determine the mRNA levels and promoter methylation status of PCDH17 in NPC cell
lines as well as 42 NPC patient specimens, we performed reverse transcription
PCR, methylation-specific PCR, and bisulfite genome sequencing. The effects of
ectopic PCDH17 expression
in NPC cell lines were determined by colony formation, cell proliferation,
wound healing, in vitro human umbilical vein endothelial cells tube formation,
migration, invasion, cell cycle, and apoptosis assays and an in vivo subcutaneous
tumor model.
Results: PCDH17 expression
was almost absent or significantly reduced in 100% of the NPC cell lines (5/5).
However, 5-aza-2′-deoxycytidine and trichostatin A treatment restored PCDH17 expression.
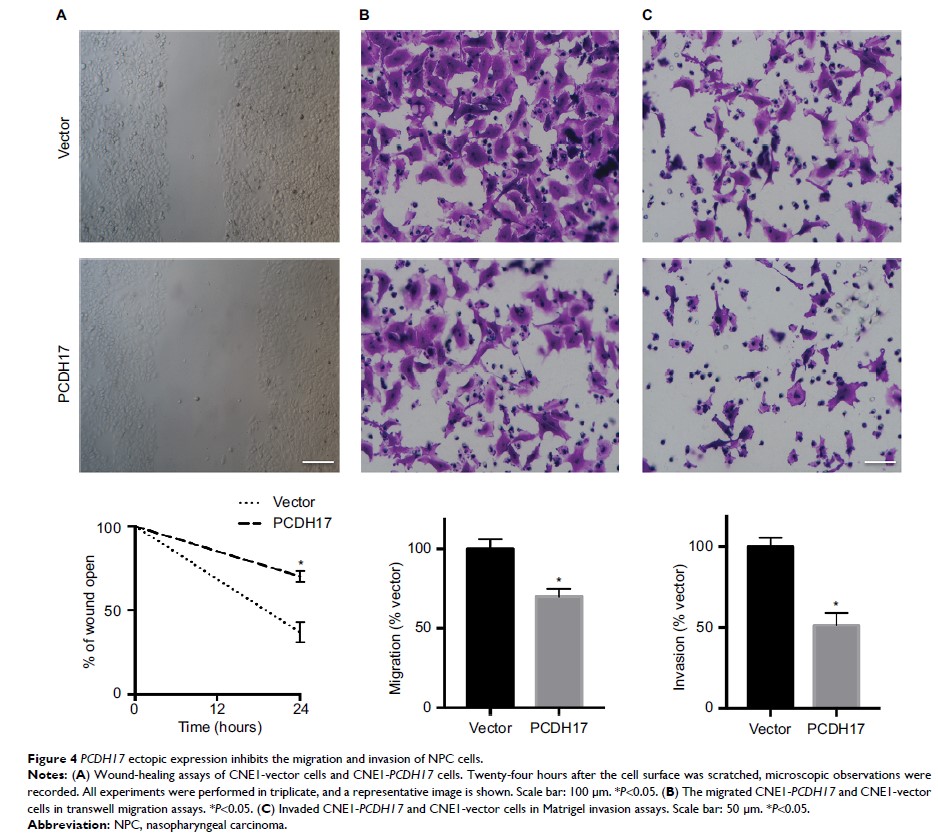
Promoter methylation was involved in PCDH17 silencing. Ectopic expression of PCDH17 in
silenced NPC cells reduced colony formation, cell migration, angiogenesis, VEGF
secretion, and tumorigenicity.
Conclusion: PCDH17 plays a
tumor suppressor role in NPC. PCDH17 methylation may be a tumor-specific event
and can be used as an epigenetic biomarker for NPC.
Keywords: nasopharyngeal
carcinoma, PCDH17 ,
tumor suppressor gene, methylation, epigenetic inactivation