109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
对中国多发性骨髓瘤患者使用来那度胺 (Lenalidomide) 的临床实用性和为病人进行的考虑
Authors Wang J, Guo H, Zhou X
Published Date June 2015 Volume 2015:8 Pages 1277—1284
DOI http://dx.doi.org/10.2147/OTT.S65762
Received 6 February 2015, Accepted 30 April 2015, Published 2 June 2015
Approved for publication by Dr Faris Farassati
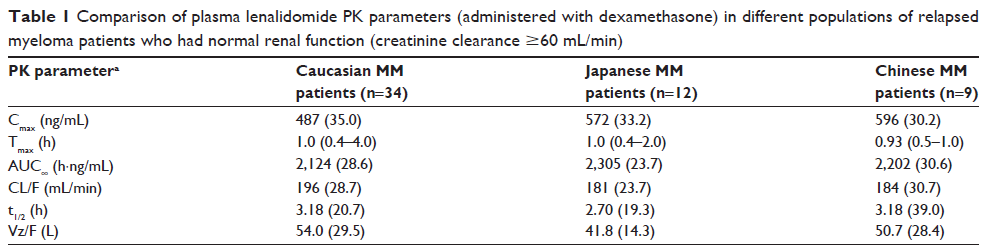
Abstract: Multiple myeloma (MM) is an incurable hematologic malignancy caused by the autonomous growth of malignant plasma cells. In the last decade, the introduction of novel targeted agents such as thalidomide, bortezomib, and lenalidomide has dramatically improved the clinical outcome of MM patients in both the frontline and recurrent settings. Lenalidomide is a synthetic derivative of thalidomide, which has been shown to significantly improve overall survival, time to progression, and overall response rates in patients with MM. The China Food and Drug Administration approved the use of lenalidomide in patients with MM in 2013. In a Phase II trial, lenalidomide plus low-dose dexamethasone was associated with a high response rate and acceptable safety profile in heavily pretreated Chinese patients with relapsed/refractory MM, including those with renal impairment and IgD subtype. However, lenalidomide will remain as a second-line antimyeloma drug in the near future because of its high price and the policy of health insurance reimbursement in People’s Republic of China. In this review, we summarize the clinical utility and patient considerations in the use of lenalidomide for MM in Chinese patients. Further studies with larger sample sizes are required to investigate the better quality, longer duration, and more clinically meaningful outcomes of lenalidomide in the treatment of MM in Chinese patients.
Keywords: lenalidomide, multiple myeloma, clinical efficacy, Chinese patients
Keywords: lenalidomide, multiple myeloma, clinical efficacy, Chinese patients