108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

对晚期或放射性碘难治性分化型甲状腺癌患者进行 TKI 治疗的相关不良反应:系统评价和荟萃分析
Authors Yu S, Ge J, Luo J, Wei Z, Sun B, Lei S
Received 19 October 2018
Accepted for publication 21 January 2019
Published 14 February 2019 Volume 2019:11 Pages 1525—1532
DOI https://doi.org/10.2147/CMAR.S191499
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Professor Luzhe Sun
Background: Tyrosine
kinase inhibitors (TKIs) have been administered to advanced or radioiodine
refractory differentiated thyroid carcinoma (RR-DTC) patients for years. We
performed a pooled analysis to explore the frequency of severe adverse effects
in advanced or RR-DTC patients treated with sorafenib and lenvatinib.
Methods: We
performed a comprehensive search of computerized databases, including PubMed,
Web of Science, Ovid, EMASE, and the Cochrane Library, from the drugs’
inception to July 2018 to identify clinical trials. All grade and severe
adverse events (AEs; grade ≥3) were analyzed. This meta-analysis was conducted
in accordance with PRISMA guidelines.
Results: In total,
seve studies published from 2012–2018 with 657 patients were eligible for this
study. We included two studies (238 patients) that received 200 mg sorafenib
twice and five studies (419 patients) that received 24 mg lenvatinib daily. The
frequency of AEs was different among the two drugs. Patients in the sorafenib
group had a significantly higher frequency of all grade hand-foot syndrome,
hypocalcemia, rash, elevated alanine aminotransferase (ALT), and elevated
aspartate aminotransferase (AST). Conversely, the lenvatinib group experienced
more frequent all grade voice change, hypertension, nausea, and vomiting compared
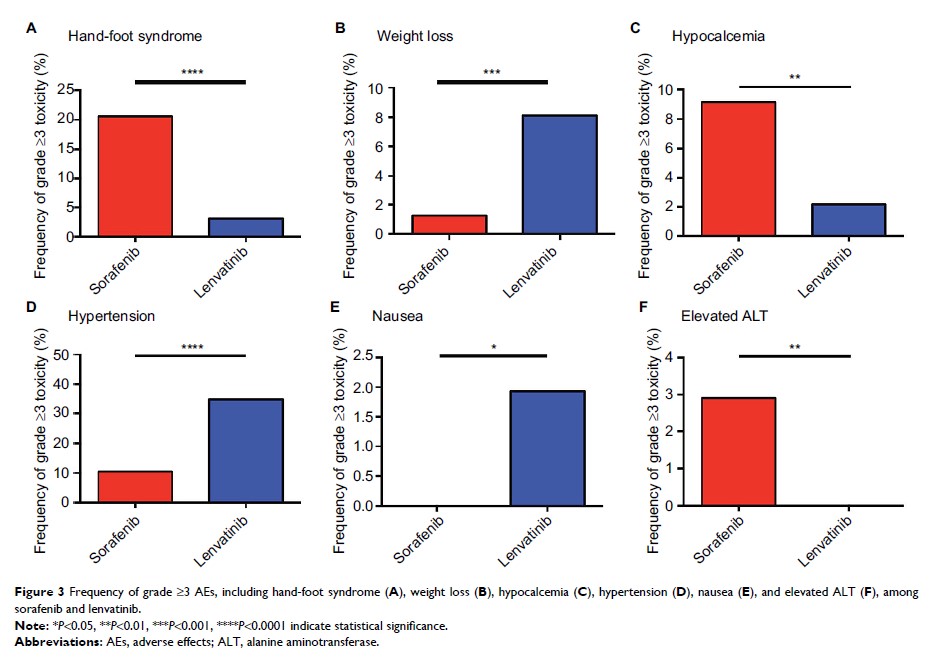
with those with sorafenib. For grade ≥3 adverse effects, hand-foot syndrome,
hypocalcemia, and elevated ALT were more frequent in sorafenib-treated
patients. Moreover, lenvatinib-treated patients had a significantly higher
incidence of severe weight loss, hypertension, and nausea.
Conclusion: Significant
differences in common adverse effects, such as all-grade and severe AEs, were
detected between sorafenib and lenvatinib in the current study. Early
intervention and management of treatment-related AEs (TRAEs) can minimize the
impact on patients’ quality-of-life, and avoid unnecessary dose reductions and
treatment-related discontinuations.
Keywords: sorafenib,
lenvatinib, radioiodine-refractory differentiated thyroid carcinoma, RR-DTC,
tyrosine kinase inhibitors, TKIs, adverse effects