108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

静脉注射氟比洛芬酯和纳布啡的术中给药方式减少了行眼眶减压术后的疼痛:一项单中心、前瞻性随机对照试验
Authors Ye H, Lian X, Chen R, Zhu Y, Chen H, Huang J, Xie L, Ma W, Yang H, Guo W
Received 16 September 2018
Accepted for publication 17 January 2019
Published 14 February 2019 Volume 2019:12 Pages 659—665
DOI https://doi.org/10.2147/JPR.S187020
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Michael Ueberall
Purpose: This
study aimed to investigate postoperative analgesia achieved with intraoperative
administration of intravenous flurbiprofen axetil and nalbuphine in patients
undergoing orbital decompression.
Methods: Sixty-three
patients undergoing orbital decompression under general anesthesia at the
Zhongshan Ophthalmic Center at Sun Yat-sen University (Guangzhou, China) were
randomly allocated into one of the following three groups (1:1:1):
intraoperative flurbiprofen axetil 100 mg (Group 1); intraoperative nalbuphine
0.1 mg/kg (Group 2); or intraoperative flurbiprofen axetil 100 mg combined with
nalbuphine 0.1 mg/kg (Group 3). The primary end point was mean postoperative
pain intensity during the first 24 hours. The secondary efficacy end points
were the intensity of pain and discomfort at 0, 2, 6, 10, and 24 hours after
surgery and side effects at 24 hours after surgery.
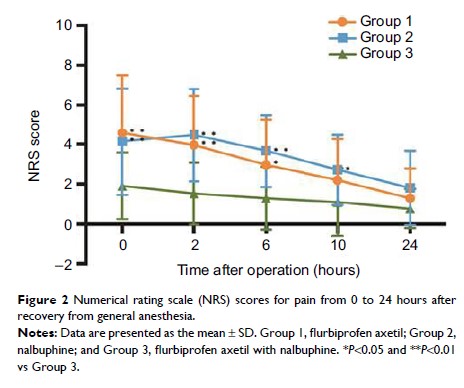
Results: The
demographic characteristics were similar among the three groups. Mean and peak
postoperative pain scores during the first 24 hours in Group 3 were lower than
those in Group 1 (P =0.007 and P =0.003, respectively) and Group 2 (P =0.001 and P =0.000,
respectively). Additionally, the pain scores in Group 3 were significantly
lower than those in Group 1 during the first 6 hours after surgery (P =0.003, 0.002, and
0.022 at 0, 2, and 6 hours, respectively) and those in Group 2 during the
first 10 hours after surgery (P =0.008, 0.000, 0.001, and 0.019 at 0, 2, 6, and
10 hours, respectively). Discomfort scores were not significantly
different among the three groups during the observation period, except at 2
hours after surgery, at which time the scores in Group 3 were significantly
lower than those in Group 2 (P =0.033). Postoperative adverse effects and analgesic
requirements were similar among the three groups.
Conclusion: Intraoperative
administration of a combination of intravenous flurbiprofen axetil and
nalbuphine is superior to single-dose flurbiprofen axetil or nalbuphine in
patients undergoing orbital decompression.
Keywords: postoperative
pain, flurbiprofen axetil, nalbuphine, orbital decompression