108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Increased dermal expression of chromatin-associated protein HMGB1 and concomitant T-cell expression of the DNA RAGE in patients with psoriasis vulgaris
Authors Strohbuecker L, Koenen H, van Rijssen E, van Cranenbroek B, Fasse E, Joosten I, Körber A, Bergmann C
Received 11 October 2018
Accepted for publication 11 January 2019
Published 12 February 2019 Volume 2019:9 Pages 7—17
DOI https://doi.org/10.2147/PTT.S190507
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Professor Uwe Wollina
Purpose: Psoriasis
vulgaris (PV) is an autoimmune-related chronic inflammatory disease of the
skin, with both vascular and metabolic effects. Aggravating factors have been
identified that initiate and maintain inflammation, including expression of
Th1-, Th17-, and Th22-cell derived cytokines. Recently, we showed that the evolutionarily
ancient and highly conserved damage-associated molecular pattern molecule “high
mobility group box 1 (HMGB1)” is significantly increased in the serum of PV
patients with disease progression and is decreased under standard therapies.
Materials and methods: To better
understand the role of HMGB1 in the pathogenesis of PV, we recruited 22
untreated psoriatic patients with either mild or severe disease, defined by the
Psoriasis Area Severity Index. We assessed HMGB1 and receptor for advanced
glycation end products (RAGE) expression in the skin by immunohistochemistry
and analyzed the immune-phenotype of Treg and Th17 cells by flow cytometry.
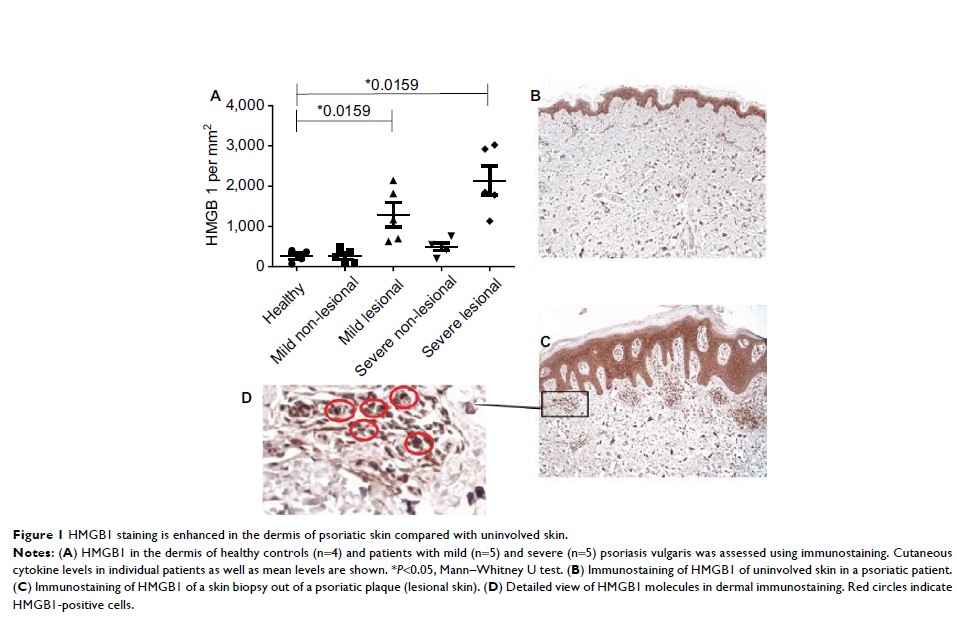
Results: We found
increased staining for HMGB1 in the dermis of psoriatic plaques in comparison
to uninvolved skin of patients with PV. In addition, the major
histocompatibility complex class III-encoded DNA and HMGB1 RAGE, induced by
HMGB1, were highly expressed on psoriatic CD8+ T cells and CD4+ Treg. High
expression of HMGB1 in the lesional skin was associated with even higher
expression of its receptor, RAGE, on the cell surface of keratinocytes in
patients with severe PV.
Conclusion: The
presence of HMGB1 and RAGE signaling may impact orchestration of chronic
inflammation in PV which might have implications for Treg and Th17 cells.
Keywords: HMGB1,
RAGE, psoriasis vulgaris, Th17