108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

CHFR 通过以 HDAC1 依赖性方式诱导上皮 - 间质转化来促进人胃癌细胞的迁移
Authors Yang S, He F, Dai M, Pan J, Wang J, Ye B
Received 15 October 2018
Accepted for publication 4 January 2019
Published 7 February 2019 Volume 2019:12 Pages 1075—1084
DOI https://doi.org/10.2147/OTT.S191016
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 4
Editor who approved publication: Dr William Cho
Background: Previous
studies have illustrated that checkpoint with forkhead-associated and ring
finger domains (CHFR) was frequently silenced in several cancer types due to
promoter hypermethylation and functions as a tumor suppressor gene. However,
the data from the public dataset reveal that CHFR is highly expressed in human
gastric cancer specimens, and the biological function of CHFR in gastric cancer
is still not well understood.
Materials and methods: The
clinical association between CHFR expression and the overall survival of
gastric cancer patients as well as cancer metastasis was analyzed according to
public datasets. The CHFR expression in clinical specimens and human gastric
cancer cell lines was detected by immunohistochemistry and Western blotting,
respectively. Gain (overexpression) and loss (silencing) of function
experiments were used to elucidate the role of CHFR in gastric cancer. The
migration ability of gastric cancer cells was determined by wound healing and
transwell assays. Cell cycle distribution was analyzed using
fluorescence-activated cell sorting experiment. The expression of the proteins
in cancer cells was measured using Western blot analysis.
Results: According
to the analysis from Kaplan–Meier plotter dataset, CHFR expression was
negatively associated with overall survival of gastric cancer patients. Our
data revealed that exogenous expression of CHFR not only arrested cell cycle
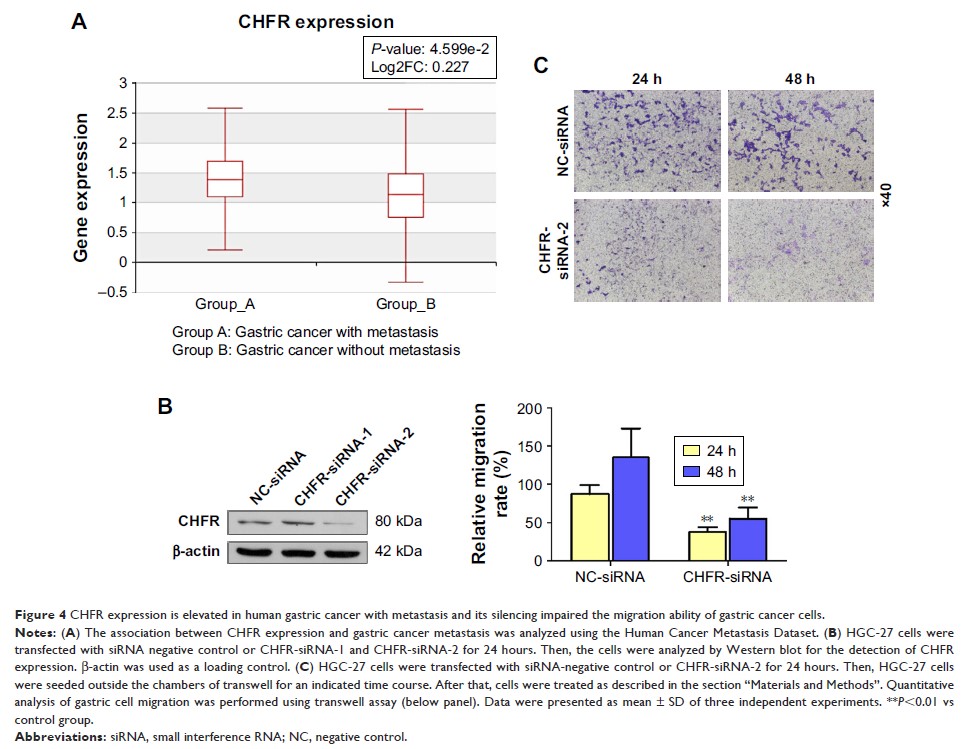
but also led to dramatically enhanced cell migration, while silencing of CHFR
significantly inhibited cell migration in gastric cancer cells. This result is
consistent with the data from the Human Cancer Metastasis Dataset, in which
CHFR level is found to significantly increase in metastatic gastric cancer. The
overexpression of CHFR promoted epithelial–mesenchymal transition (EMT) in both
SGC-7901 and AGS cells, while HDAC1 was inhibited. Interestingly,
suberoylanilide hydroxamic acid, a HDAC1 antagonist, could effectively increase
cell migration in both cell lines via enhancement of EMT.
Conclusion: Our data
indicated that CHFR exerted positive effects on cell migration of human gastric
cancer by promoting EMT via downregulating HDAC1.
Keywords: human
gastric cancer, migration, CHFR, EMT, HDAC1