109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

应用超临界流体技术制备伊曲康唑 (Itraconazole) 固体分散体 制备、体外表征和用于比格犬的生物药效率
Authors Yin X, Daintree LS, Ding S, Ledger DM, Wang B, Zhao W, Qi J, Wu W
Published Date May 2015 Volume 2015:9 Pages 2801—2810
DOI http://dx.doi.org/10.2147/DDDT.S81253
Received 20 January 2015, Accepted 19 March 2015, Published 28 May 2015
Abstract: This
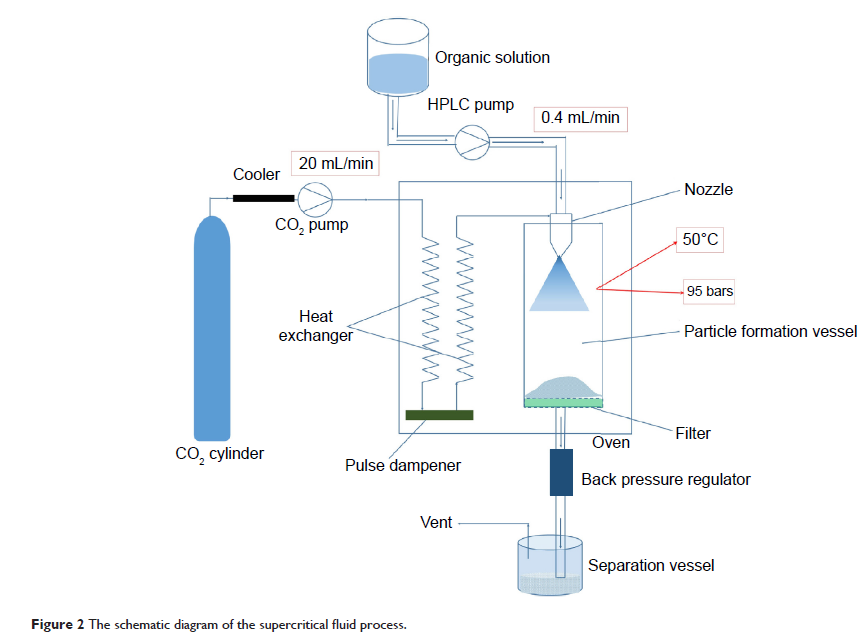
research aimed to develop a supercritical fluid (SCF) technique for preparing a
particulate form of itraconazole (ITZ) with good dissolution and
bioavailability characteristics. The ITZ particulate solid dispersion was
formulated with hydroxypropyl methylcellulose, Pluronic F-127, and l-ascorbic
acid. Aggregated particles showed porous structure when examined by scanning
electron microscopy. Powder X-ray diffraction and Fourier transform infrared
spectra indicated an interaction between ITZ and excipients and showed that ITZ
existed in an amorphous state in the composite solid dispersion particles. The solid
dispersion obtained by the SCF process improved the dissolution of ITZ in media
of pH 1.0, pH 4.5, and pH 6.8, compared with a commercial product
(Sporanox®), which could be ascribed to the porous aggregated
particle shape and amorphous solid state of ITZ. While the solid dispersion did
not show a statistical improvement (P =0.50) in terms
of oral bioavailability of ITZ compared with Sporanox®, the C max (the maximum plasma
concentration of ITZ in a pharmacokinetic curve) of ITZ was raised
significantly (P =0.03) after oral administration.
Thus, the SCF process has been shown to be an efficient, single step process to
form ITZ-containing solid dispersion particles with good dissolution and oral
bioavailability characteristics.
Keywords: gas anti-solvent, dissolution,
HPMC, Pluronic F-127, ascorbic acid, in vivo