108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

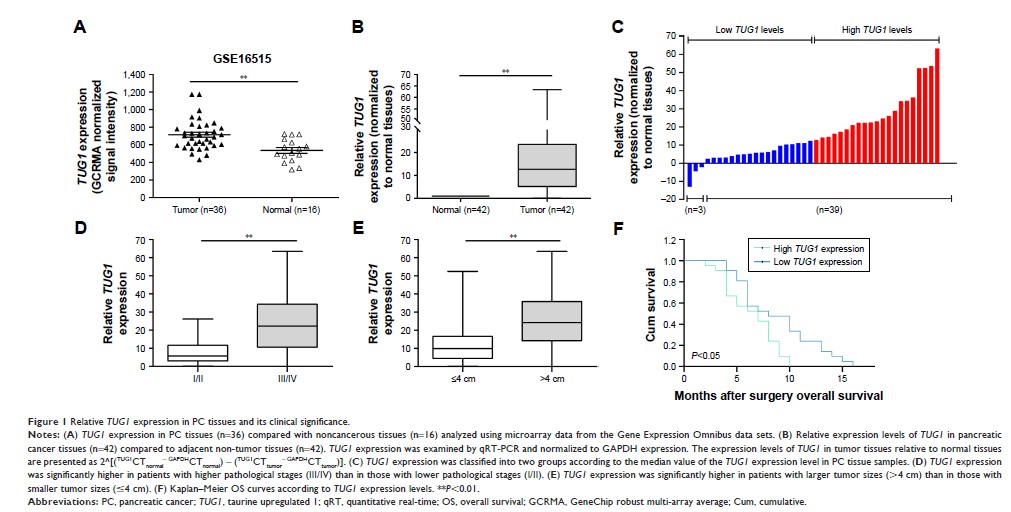
过表达的长非编码 RNA TUG1 通过抑制 RND3 和 MT2A 部分地影响胰腺癌的细胞周期、增殖和凋亡
Authors Hui B, Xu Y, Zhao B, Ji H, Ma Z, Xu S, He Z, Wang K, Lu J
Received 21 September 2018
Accepted for publication 9 January 2019
Published 5 February 2019 Volume 2019:12 Pages 1043—1057
DOI https://doi.org/10.2147/OTT.S188396
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Background: Long
noncoding RNAs (lncRNAs) are involved in various human diseases, including
cancers. However, their mechanisms remain undocumented. We investigated
alterations in lncRNA that may be related to pancreatic cancer (PC) through
analysis of microarray data.
Methods: In the
present study, quantitative real-time PCR analysis was used to examine the
expression of taurine upregulated 1 (TUG1 ) in PC tissue samples and PC cell lines. In PC
cell lines, MTT assays, colony formation assays, and flow cytometry were used
to investigate the effects of TUG1 on proliferation, cell cycle regulation, and
apoptosis. Moreover, we established a xenograft model to assess the effect
of TUG1 on
tumor growth in vivo. The molecular mechanism of potential target genes was
detected through nuclear separation experiments, RNA immunoprecipitation (RIP),
chromatin immunoprecipitation assays (ChIP), and other experimental methods.
Results: The
findings suggest that the abnormally high expression of TUG1 in PC
tissues was associated with tumor size and pathological stage. Knockdown
of TUG1 blocked
the cell cycle and accelerated apoptosis, thereby inhibiting the proliferation
of PC cells. In addition, RIP experiments showed that TUG1 can
recruit enhancer of zeste homolog 2 (EZH2) to the promoter regions of Rho
family GTPase 3 (RND3 ) and metallothionein 2A (MT2A ) and inhibit
their expression at the transcriptional level. Furthermore, ChIP experiments
demonstrated that EZH2 could bind to the promoter regions of RND3 and MT2A . The knockdown
of TUG1 reduced
this binding capacity.
Conclusion: In
conclusion, our data suggest that TUG1 may regulate the expression of
PC-associated tumor suppressor genes at the transcriptional level and these may
become potential targets for the diagnosis and treatment of PC.
Keywords: LncRNA,
ncRNA, regulate, mechanism, cancer, EZH2, transcriptional level, tumor
suppressor genes