108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

sh-HIF1A-AS2 通过靶向 miR-548c-3p,经由体外和体内 HIF-1α/VEGF 通路调控对乳腺癌细胞增殖、侵袭和病理损伤
Authors Guo X, Lee S, Cao P
Received 26 October 2018
Accepted for publication 25 December 2018
Published 24 January 2019 Volume 2019:12 Pages 825—834
DOI https://doi.org/10.2147/OTT.S192377
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Background: Breast
cancer (BC) has been the commonest malignant tumor with a low survival rate among
woman. Long non-coding RNA hypoxia-inducible factor-1 alpha antisense RNA-2
(HIF1A-AS2) was correlated with various cancers.
Purpose: The study
aimed to investigate the roles and related underlying molecular mechanisms of
HIF1A-AS2 in BC.
Material and methods: Target
relationships were speculated by Targetscan 7.0 and confirmed by dual
luciferase reporter assay. Proteins levels were monitored by RT-qPCR, Western
blot and immunohistochemistry assays. CCK-8 assay, SA-β-gal staining and
transwell assay were used to detect proliferation, senescence and invasion,
respectively. Xenograft nude mice were put into use to evaluate the tumor
growth and motility.
Results: The
present study exhibited that HIF1A-AS2 and hypoxia-inducible factor-1 alpha
(HIF-1α) were upregulated while miR-548c-3p was downregulated in MDA-MB-231,
MCF-7, ZR-75-1, and BT-549 BC cell lines. Bioinformatics analysis showed
HIF1A-AS2 and HIF-1α were two targets of miR-548c-3p, and the target
relationship was further confirmed by dual luciferase reporter assay. Moreover,
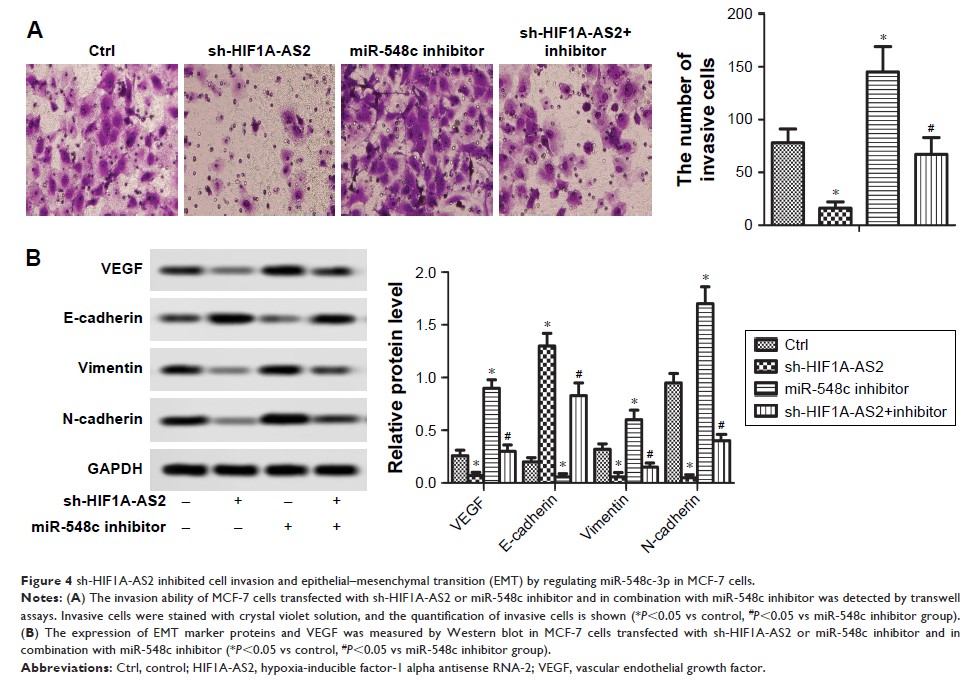
knockdown of HIF1A-AS2 by shRNA (sh-HIF1A-AS2) markedly elevated miR-548c-3p
level, and the enhanced miR-548c-3p noticeably suppressed cell proliferation,
invasion, and epithelial–mesenchymal transition, and promoted senescence
in vitro. In addition, overexpression of HIF-1α promoted MCF-7 cell
invasion. Intriguingly, low expression of HIF1A-AS2 reduced HIF-1α level by
upregulating the expression of miR-548c-3p. Furthermore, experiment in
xenograft nude mice has indicated that sh-HIF1A-AS2 inhibited tumor growth and
motility by targeting miR-548c-3p through regulating HIF-1α/vascular
endothelial growth factor (VEGF) pathway in vivo.
Conclusion: The
inhibitive effect of HIF-1α/VEGF pathway by sh-HIF1A-AS2 through targeting
miR-548c-3p plays crucial regulatory roles in BC. Therefore, designing targeted
drugs against HIF1A-AS2 provides a new direction for the treatment of BC.
Keywords: breast
cancer, oncogenesis, HIF1A-AS2, miR-548c-3p, HIF-1α/VEGF, MCF-7