109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
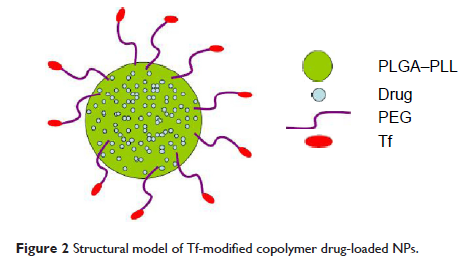
转铁蛋白 (Transferrin) 修饰的肿瘤靶向共聚物纳米载体的合成和特征描述
Authors Liu R, Wang Y, Li X, Bao W, Xia G, Chen W, Cheng J, Xu Y, Guo L, Chen B
Published Date May 2015 Volume 2015:9 Pages 2705—2719
DOI http://dx.doi.org/10.2147/DDDT.S80948
Received 15 January 2015, Accepted 18 March 2015, Published 22 May 2015
Abstract: To increase the encapsulation of hydrophilic antitumor agent
daunorubicin (DNR) and multidrug resistance reversal agent tetrandrine (Tet) in
the drug delivery system of nanoparticles (NPs), a functional copolymer NP
composed of poly(lactic-co-glycolic acid) (PLGA), poly-l-lysine (PLL), and
polyethylene glycol (PEG) was synthesized and then loaded with DNR and Tet
simultaneously to construct DNR/Tet–PLGA–PLL–PEG-NPs using a modified
double-emulsion solvent evaporation/diffusion method. And to increase the targeted
antitumor effect, DNR/Tet–PLGA–PLL–PEG-NPs were further modified with
transferrin (Tf) due to its specific binding to Tf receptors (TfR), which is
highly expressed on the surface of tumor cells. In this study, the influence of
the diversity of formulation parameters was investigated systematically, such
as drug loading, mean particle size, molecular weight, the concentration of
PLGA–PLL–PEG–Tf, volume ratio of acetone to dichloromethane, the concentration
of polyvinyl alcohol (PVA) in the external aqueous phase, the volume ratio of
the internal aqueous phase to the external aqueous phase, and the type of
surfactants in the internal aqueous phase. Meanwhile, its possible effect on
cell viability was evaluated. Our results showed that the regular spherical
DNR/Tet–PLGA–PLL–PEG–Tf-NPs with a smooth surface, a relatively low
polydispersity index, and a diameter of 213.0±12.0 nm could be produced. The
encapsulation efficiency was 70.23%±1.91% for DNR and 86.5%±0.70% for Tet, the
moderate drug loading was 3.63%±0.15% for DNR and 4.27%±0.13% for Tet. Notably,
the accumulated release of DNR and Tet could be sustained over 1 week, and the
Tf content was 2.18%±0.04%. In cell viability tests,
DNR/Tet–PLGA–PLL–PEG–Tf-NPs could inhibit the proliferation of K562/ADR cells
in a dose-dependent manner, and the half maximal inhibitory concentration value
(total drug) of DNR/Tet–PLGA–PLL–PEG–Tf-NPs was lower than that of DNR, a
mixture of DNR and Tet, and DNR/Tet–PLGA–PLL–PEG-NPs. These results clearly
indicate that the PLGA–PLL–PEG formulation is a potential drug delivery system
for hydrophilic and hydrophobic drugs, and that Tf modification may increase
its targeting properties.
Keywords: PLGA, PLL, PEG, daunorubicin, tetrandrine
Keywords: PLGA, PLL, PEG, daunorubicin, tetrandrine