109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

结合帕尼单抗 (Panitumumab) 和贝伐单抗 (bevacizumab),加上伊利替康 (irinotecan),5 氟尿嘧啶和亚叶酸钙(FOLFIRI 疗法)的综合疗法,与单独使用FOLFIRI 疗法相比,作为对转移性结肠直肠癌和柯尔斯顿鼠肉瘤病毒癌基因 (KRAS) 突变患者的二线治疗的一个随机 II 期临床研究
Authors Liu YG, Luan LJ, Wang XL
Published Date May 2015 Volume 2015:8 Pages 1061—1068
DOI http://dx.doi.org/10.2147/OTT.S81442
Received 23 January 2015, Accepted 17 March 2015, Published 14 May 2015
Background: This study investigated the efficacy and
safety of a new treatment strategy of combining panitumumab and bevacizumab,
plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) versus FOLFIRI alone
as second-line chemotherapy for metastatic colorectal cancer (mCRC) patients
with known V-Ki-ras2 Kirsten rat sarcoma viral oncogene (KRAS ) mutation status.
Methods: Patients with mCRC who
had known KRAS tumor status and unsuccessful previous oxaliplatin-based
chemotherapy were included in the study. They were randomly assigned to two
groups to receive panitumumab and bevacizumab plus FOLFIRI, or FOLFIRI alone.
In panitumumab and bevacizumab plus FOLFIRI group, patients were given 4 mg/kg
panitumumab and bevacizumab plus FOLFIRI every 2 weeks.
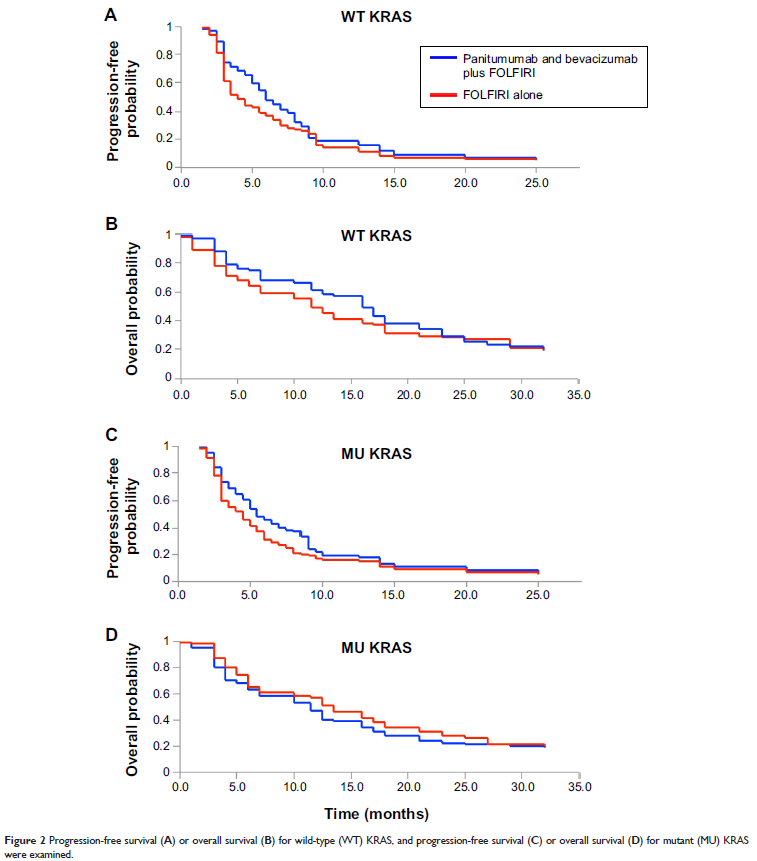
Results: In all, 65 patients
were assigned to panitumumab and bevacizumab plus FOLFIRI group, and 77 to
FOLFIRI alone group. For WT KRAS patients, the median progression-free survival
(PFS) was 5.7 months (95% confidence interval [CI], 2.4–7.5 months) for
panitumumab and bevacizumab plus FOLFIRI and 3.8 months (95% CI, 3.0–6.7
months) for FOLFIRI alone; median overall survival (OS) was 15.2 months (95%
CI, 8.9–19.7 months) for panitumumab and bevacizumab plus FOLFIRI and 11.0
months (95% CI, 8.2–15.4 months) for FOLFIRI alone. For MU KRAS patients,
median PFS was 5.1 months (95% CI, 2.7–10.2 months) for panitumumab and
bevacizumab plus FOLFIRI and 4.1 months (95% CI, 2.5–8.4 months) for FOLFIRI
alone; median OS was 12.8 months (95% CI, 7.8–15.8 months) for panitumumab and
bevacizumab plus FOLFIRI and 10.5 months (95% CI, 6.1–15.3 months) for FOLFIRI
alone. Grade 3 and 4 adverse events were associated with panitumumab and
bevacizumab plus FOLFIRI but tolerable among patients.
Conclusion: Patients with mCRC
can be safely and efficiently treated with second-line chemotherapy of
combining panitumumab and bevacizumab plus FOLFIRI, despite their KRAS mutation
status.
Keywords: metastatic colorectal
cancer, panitumumab, bevacizumab, FOLFIRI, second-line chemotherapy