109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

对树枝状壳聚糖衍生物在红细胞中的血液相容性研究
Authors Zhou YF, Li JM, Lu F, Deng JJ, Zhang JH, Fang PJ, Peng XS, Zhou SF
Published Date May 2015 Volume 2015:9 Pages 2635—2645
DOI http://dx.doi.org/10.2147/DDDT.S77105
Received 6 November 2014, Accepted 18 March 2015, Published 14 May 2015
Abstract: Dendrimers
are hyperbranched macromolecules with well-defined topological structures and
multivalent functionalization sites, but they may cause cytotoxicity due to the
presence of cationic charge. Recently, we have introduced alkyne-terminated
poly(amidoamine) (PAMAM) dendrons of different generations (G=2,3) into
chitosan to obtain dendronized chitosan derivatives [Cs-g-PAMAM (G=2,3)], which exhibited a
better water solubility and enhanced plasmid DNA transfection efficiency. In
this study, we attempted to examine the impact of Cs-g -PAMAM (G=2,3) at different
concentrations (25 µg/mL, 50 µg/mL, and 100 µg/mL) on the
morphology, surface structure, and viability of rat red blood cells (RBCs). The
results showed that treatment of RBCs with Cs-g -PAMAM
(G=2,3) at 50 µg/mL and 100 µg/mL induced a slightly higher hemolysis
than Cs, and Cs-g -PAMAM (G=3) caused a slightly
higher hemolysis than Cs-g -PAMAM (G=2),
but all values were <5.0%. Optical microscopic and atomic force microscopic
examinations indicated that Cs-g -PAMAM (G=2,3)
caused slight RBC aggregation and lysis. Treatment of RBCs with 100 µg/mL
Cs-g -PAMAM (G=3) induced echinocytic
transformation, and RBCs displayed characteristic irregular contour due to the
folding of the periphery. Drephanocyte-like RBCs were observed when treated
with 100 µg/mL Cs-g -PAMAM (G=3).
Erythrocytes underwent similar shape transition upon treatment with Cs-g -PAMAM (G=2) or Cs. The roughness
values (Rms) of RBCs incubated with Cs-g -PAMAM (G=2,3)
were significantly larger than those for RBCs incubated with physiological
saline (P <0.01), but the Rms showed no
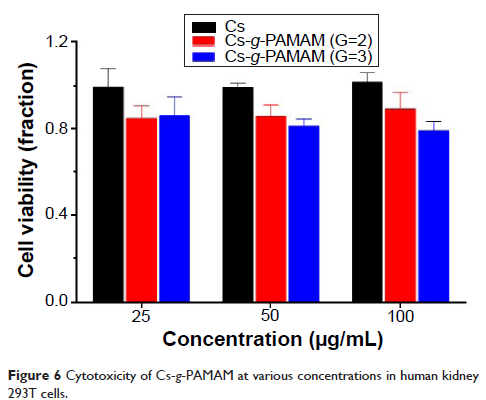
difference for Cs and Cs-g -PAMAM (G=2,3) (P >0.05). Furthermore, Cs-g -PAMAM (G=2,3) exhibited a lower cytotoxicity
in human kidney 293T cells. These results indicate that Cs-g -PAMAM (G=2,3) are hemocompatible
but may disturb membrane and lipid structures at higher concentrations.
Further safety and biocompatibility evaluations are warranted for Cs-g -PAMAM. Our findings prove helpful
for a better understanding of the advantages of combining PAMAM dendrimers and
chitosan to design and develop new, safe, and effective drug delivery vehicles.
Keywords: dendronized chitosan
derivative, PAMAM, RBC, hemolysis, hemocompatibility