108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

埃克替尼治疗后有好转的中国非小细胞肺癌患者的再活检状态
Authors Wang H, Zhang L, Si X, Zhang X, Wang M
Received 14 May 2018
Accepted for publication 17 September 2018
Published 26 October 2018 Volume 2018:11 Pages 7513—7519
DOI https://doi.org/10.2147/OTT.S174075
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Objective: Acquired T790M mutations account for 50%–60% of tyrosine kinase inhibitor (TKI)-resistant mechanisms in EGFR mutation-positive (m+) non-small-cell lung cancer (NSCLC) patients, and re-biopsy is recommended to detect these mutations. We investigated the re-biopsy status and the T790M incidence rate in patients after treatment with icotinib, which is the first-generation EGFR-TKI widely used in China.
Patients and methods: Target patients had EGFRm+NSCLC, who were progressed after icotinib therapy. The primary end point was the re-biopsy rate (number of cases in which re-biopsies were performed successfully/total number of patients progressed after icotinib therapy). Secondary end points included the T790M mutation incidence rate, differences between the first biopsy and re-biopsy, and details of why re-biopsy was not performed in relevant patients.
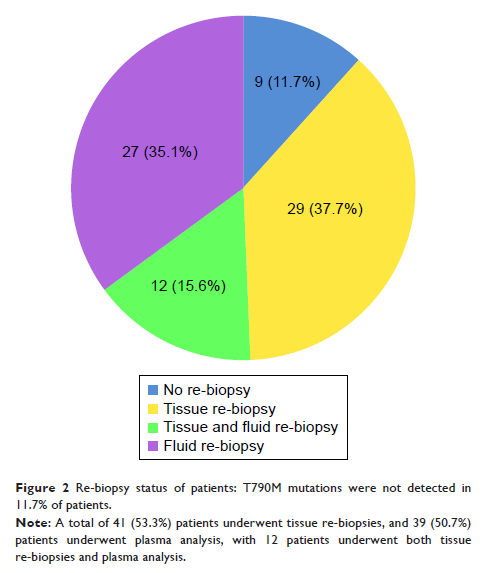
Results: A total of 77 adenocarcinoma patients were evaluated (median age, 58 years). Tissue re-biopsy was successful in 41 patients (53.2%). Compared with the first biopsy, percutaneous tissue biopsies increased from 51.2% to 70.7% (P =0.008), while bronchoscopy biopsies and the surgical rate decreased from 19.5% to 14.6% (P <0.001) and 17.1% to 7.3% (P <0.001), respectively. Primary lung lesions were more common in the first biopsy than in re-biopsy (80.5% vs 65.9%, P =0.008), but metastatic lesions were more often selected for re-biopsy (14/41 [34.1%], including metastases in the bone, lymph nodes, and liver). The incidence rate of T790M was 56.1% (23/41). The reasons for not performing re-biopsies included lesion sizes and/or locations unsuitable for biopsy (n=17), a positive circulating tumor DNA (ctDNA) result (n=3), patient unwillingness (n=7), older age or severe comorbidity (n=4), and poor health (n=5). No severe complications were found.
Conclusion: In this real-world study, the re-biopsy rate was 53.2% and the incidence rate of T790M mutations was 56.1%. Further efforts are needed to increase the re-biopsy rate in patients who progress after icotinib therapy.
Keywords: re-biopsy, T790M mutation, resistant mechanism, EGFR-TKI, icotinib, non-small-cell lung cancer