108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

基于 γ-PGA 的自组装转铁蛋白受体靶向抗癌药物载体的制备和评价
Authors Zhang L, Zhu X, Wu S, Chen Y, Tan S, Liu Y, Jiang W, Huang J
Received 23 July 2018
Accepted for publication 11 October 2018
Published 22 November 2018 Volume 2018:13 Pages 7873—7889
DOI https://doi.org/10.2147/IJN.S181121
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Thomas J. Webster
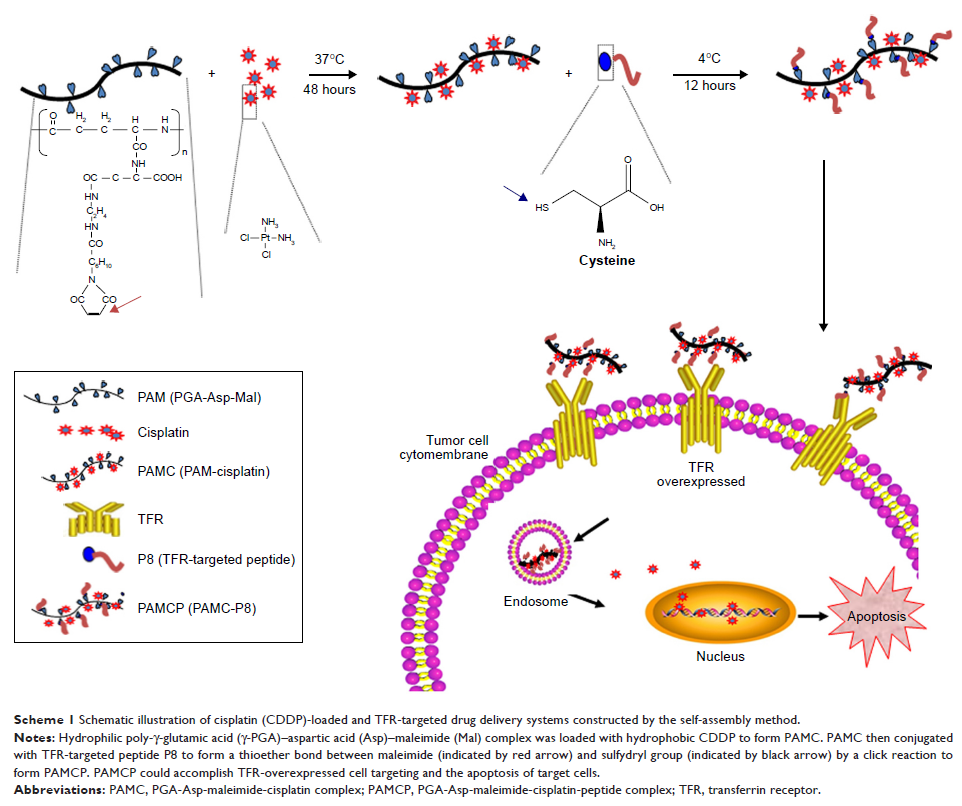
Background: cis-Dichlorodiamineplatinum (CDDP) was
one of the most common used drugs in clinic for cancer treatment. However, CDDP
caused a variety of side effects. The abundant carboxyl groups on the surface
of poly glutamic acid (PGA) could be modified with various kinds of targeted
ligands. PGA delivery system loaded CDDP for cancer therapies possesses
potential to overcome the side effects.
Materials and methods: In this study, we constructed a safe and efficient anticancer drug
delivery system PGA–Asp–maleimide–cisplatin–peptide complex (PAMCP), which was
loaded with CDDP and conjugated with the transferrin receptor (TFR)-targeting
peptide through a maleimide functional linker. The size of PAMCP was identified
by transmission electron microscopy (TEM) and dynamic light scattering (DLS).
Fluorescence microscopy and flow cytometry methods were used to detect the cell
targeting ability in vitro. The MTT assay was used to detect targeted toxicity
in vitro. The in vivo acute toxicity was tested in Kun Ming (KM) mice. The
tumor suppression activity and drug distribution was analyzed in nude mice
bearing with HeLa tumor cells.
Results: The
nano-size was 110±28 nm detected with TEM and 89±18 nm detected with DLS,
respectively. Fluorescence microscopy and flow cytometry methods indicated that
PAMCP possessed excellent cell targeting ability in vitro. The MTT assay
suggested that PAMCP was excellent for targeted toxicity. The acute in vivo
toxicity study revealed that the body mass index and serum index in the
PAMCP-treated group were superior to those in the CDDP-treated group (P <0.001), and no
obvious differences were detected on comparing with the PBS- or
PGA–Asp–maleimide–P8 (PAMP)-treated groups. PAMCP reduced the toxicity of CDDP,
suppressed tumor cell growth, and achieved efficient anti-tumor effects in
vivo. After PAMCP treatment, the toxicity of CDDP was reduced and tumor growth
was more remarkably inhibited compared with the free CDDP treatment group (P <0.01). Much
stronger (5–10 folds) fluorescence intensity in tumor tissue was detected
compared with the irrelevant-peptide group for drug distribution analysis
detected with the frozen section approach.
Conclusion: Our
data highlighted that PAMCP reduced the side effects of CDDP and exhibited
stronger anti-tumor effects. Therefore, PAMCP presented the potential to be a
safe and effective anticancer pharmaceutical formulation for future clinical
applications.
Keywords: cisplatin,
tumor suppression, side effects, targeted drug delivery system