108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

小分子修饰的仿生明胶/羟基磷灰石纳米纤维构建理想的成骨微环境可显著增强颅骨形成
Authors Li D, Zhang K, Shi C, Liu L, Yan G, Liu C, Zhou Y, Hu Y, Sun H, Yang B
Received 17 May 2018
Accepted for publication 1 October 2018
Published 6 November 2018 Volume 2018:13 Pages 7167—7181
DOI https://doi.org/10.2147/IJN.S174553
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Justinn Cochran
Peer reviewer comments 3
Editor who approved publication: Dr Lei Yang
Background: Repair of nonunion critical-sized bone defects is a significant
clinical challenge all over the world. Construction of osteogenic
microenvironment that provides osteoconductive and osteoinductive signals is a
leading strategy.
Materials and methods: In the present study, ascorbic acid (AA) and
β-glycerophosphate disodium salt hydrate (β-GP) modified biomimetic
gelatin/hydroxyapatite (GH) nanofibrous scaffolds were developed by
electrospinning. Then the scaffolds were crosslinked by
N-hydroxysulfo-succinimide sodium salt (NHS) and
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC). The
morphology of the non-crosslinked and crosslinked scaffolds was evaluated by
scanning electron microscope (SEM). Fourier transform infrared spectroscopy
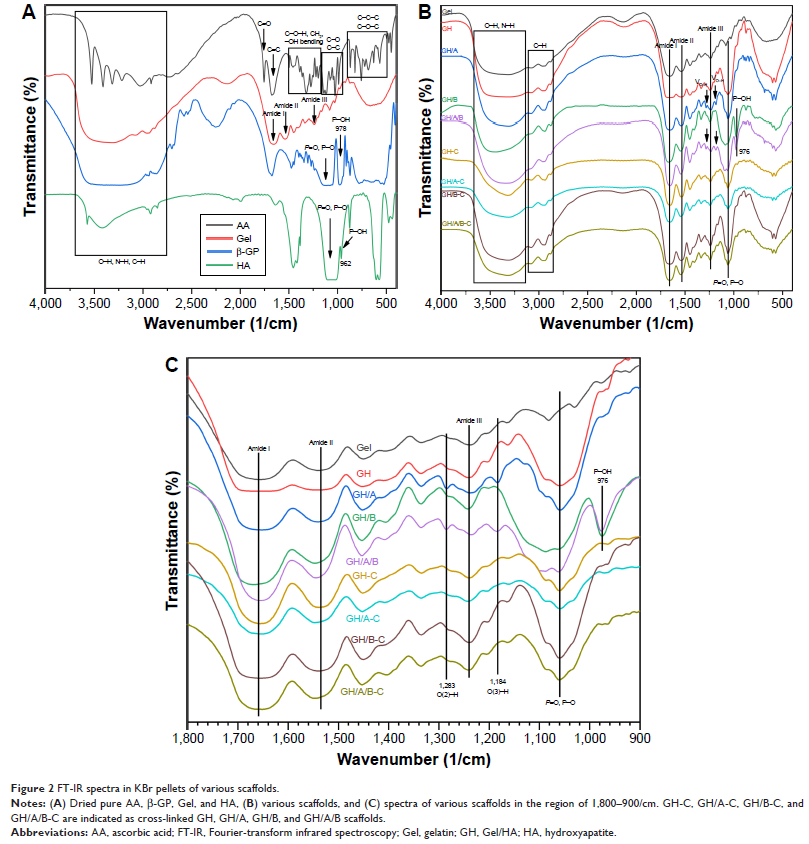
(FT-IR) was used to assess the interacting model between the small molecules and
GH scaffold. Then MTT, Alamar Blue, and CCK8 assays were used to investigate
the biocompatibility of the various crosslinked scaffolds. Subsequently, the
osteogenic genes expression of bone marrow stromal cells (BMSCs) cultured on
the scaffolds were detected by quantitative reverse transcription polymerase
chain reaction (qRT-PCR). Finally, the crosslinked scaffolds were implanted in
a rat calvarial defect model to assess the osteogenic effects in vivo.
Results: SEM results showed that the various scaffolds
presented extracellular matrix (ECM)-like fibrous porous structure. (FT-IR)
spectrum indicated that AA and β-GP were covalently bonded with GH scaffolds.
The MTT, Alamar Blue, and CCK8 assays demonstrated that all the scaffolds can
support BMSCs’ growth well. The qRT-PCR results showed that the expression
level of Alp and Runx2 in BMSCs
on GH/A/B scaffold was about 3.5- and 1.5-fold, respectively, compared with
that of GH group on day 7. The results also showed that AA- and β-GP-modified
GH scaffolds can significantly induce the higher levels of osteogenic gene
expression in a temporal specific manner. Importantly, AA and β-GP
synergistically promoted osteoblast differentiation in vitro and dramatically
induced bone regeneration in vivo. Impressively, AA and β-GP dual modified GH
nanofibrous scaffold could serve as a template for guiding bone regeneration
and the bone defects were almost repaired completely (94.28%±5.00%) at 6 weeks.
Besides, single AA or β-GP-modified GH nanofibrous scaffolds could repair 62.95%±9.39%
and 66.56%±18.45% bone defects, respectively, at 12 weeks in vivo. In addition,
AA and β-GP exhibit an anti-inflammatory effect in vivo.
Conclusion: Our data highlighted that, AA, β-GP, and GH
nanofibers created a fine osteoconductive and osteoinductive microenvironments
for bone regeneration. We demonstrated that AA and β-GP dual modified GH
nanofiber is a versatile bone tissue engineering scaffold.
Keywords: microenvironment,
bone tissue engineering, β-glycerophosphate disodium salt hydrate, ascorbic
acid, gelatin, hydroxyapatite nanofibers