108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

一种新型的由二硫键介导、可分解及 RGD 修饰的 PAMAM 纳米复合物含有核定位信号 HMGB1,用于提高基因转染效率
Authors Li J, Han Y, Lu Y, Song B, Zhao M, Hu H, Chen D
Received 3 August 2018
Accepted for publication 23 September 2018
Published 6 November 2018 Volume 2018:13 Pages 7135—7153
DOI https://doi.org/10.2147/IJN.S182445
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Background: Polyamidoamine (PAMAM) dendrimers modified by polyethylene glycol (PEG)
have frequently been investigated as a delivery carrier for gene therapy.
However, modification of PAMAM with PEG using covalent linkage significantly
reduces the cellular uptake rate and the transfection efficiency. How to
conquer these barriers becomes a burning question in gene delivery.
Materials and
methods: The present study constructed an
effective disulfide bond-mediated cleavable RGD modified gene delivery system
to overcome the aforementioned limitations. The disulfide bond was introduced between
PAMAM dendrimers and PEG chains to realize the cleavage of PEG from the carrier
system, whereas the arginine-glycine-aspartate (RGD) peptide was expected to
promote the cellular uptake rate. A high mobility group Box 1 (HMGB1) protein
containing nuclear localization signal (NLS) was simultaneously introduced to
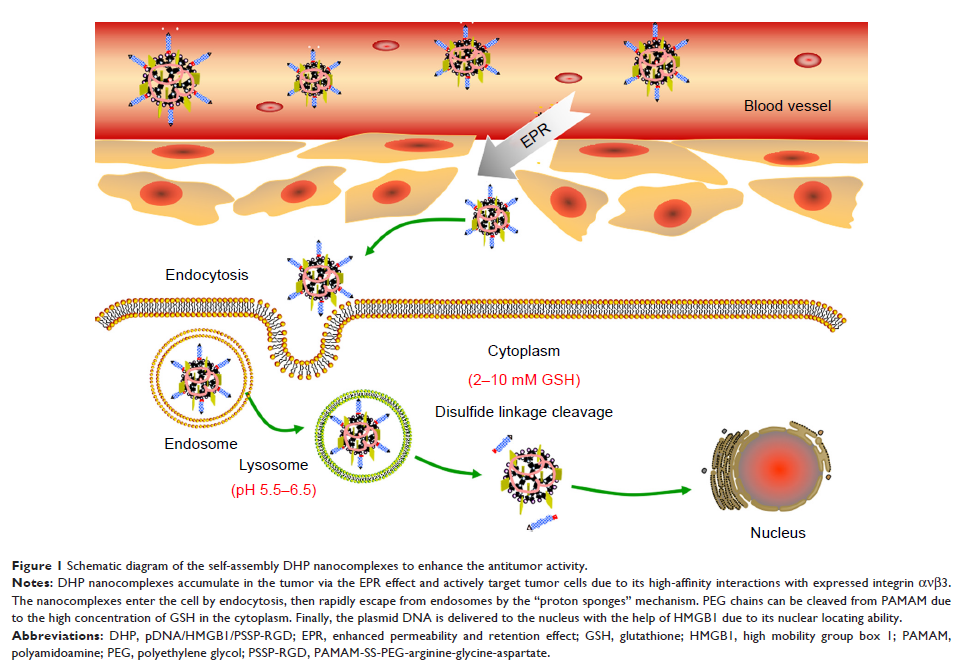
further promote gene expression efficiency. A pDNA/HMGB1/PAMAM-SS-PEG-RGD (DHP)
nanocomplex was prepared via electrostatic interaction and characterized.
Results: The results showed that DHP generated small particles and was able
to condense and protect pDNA against degradation. In addition, the RGD peptide
could significantly promote the cellular uptake of a nanocomplex. Intracellular
trafficking and in vitro expression study indicated that the DHP nanocomplex
escaped from lysosomes and the disulfide bonds between PAMAM and PEG cleaved
due to the high concentration of GSH in the cytoplasm, pDNA consequently became
exclusively located in the nucleus under the guidance of HMGB1, thereby promoting
the red fluorescence protein (RFP) expression. Importantly, an in vivo
antitumor activity study demonstrated that the DHP nanocomplex had higher
antitumor activity than any other reference preparation.
Conclusion: All these results confirm that DHP could be a new strategy for
improving the transfection and expression efficiency in gene delivery.
Keywords: PAMAM dendrimers, disulfide bond, RGD, HMGB1, gene delivery