108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

树突状细胞疫苗和由细胞因子诱导的杀伤细胞联合化疗治疗结直肠癌 荟萃分析
Authors Zhou X, Mo X, Qiu J, Zhao J, Wang S, Zhou C, Su Y, Lin Z, Ma H
Received 6 May 2018
Accepted for publication 15 August 2018
Published 5 November 2018 Volume 2018:10 Pages 5363—5372
DOI https://doi.org/10.2147/CMAR.S173201
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 3
Editor who approved publication: Professor Nakshatri
Aim: To investigate the efficacy and safety of dendritic cell (DC)
vaccine combined with cytokine-induced killer (CIK) cell therapy in colorectal
carcinoma (CRC).
Patients and
methods: PubMed, Embase, and Cochrane
Library databases were searched systematically for clinical trials of DC vaccine
and CIK cell therapy combined with chemotherapy for CRC. The primary and
secondary endpoints were overall survival (OS) and disease-free survival (DFS),
respectively. Pooled risk ratios were used to assess the treatment efficacy.
Both random and fixed effects models were used for statistical analysis. The
study population consisted of 871 CRC patients enrolled in four trials.
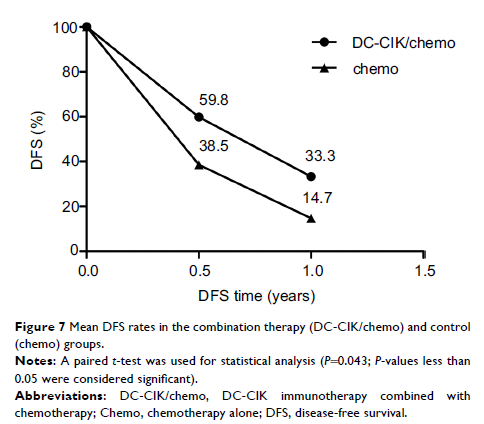
Results: OS and DFS were significantly improved in patients who received
chemotherapy combined with DC vaccine and CIK cells, and no severe adverse
events were shown.
Conclusions: The study demonstrated that the addition of DC vaccine and CIK
cell therapy to chemotherapy is feasible and effective in patients with CRC.
Keywords: adoptive cellular therapy, immunotherapy, dendritic cell vaccine,
cytokine-induced killer cell, overall survival, disease-free survival,
colorectal carcinoma