108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

鞘内注射右美托咪定对预防脊髓麻醉的剖宫产术后寒战的作用:荟萃分析和试验序贯分析
Authors Miao S, Shi M, Zou L, Wang G
Received 29 June 2018
Accepted for publication 9 October 2018
Published 2 November 2018 Volume 2018:12 Pages 3775—3783
DOI https://doi.org/10.2147/DDDT.S178665
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Objective: Intrathecal dexmedetomidine (DEX) has been used to prevent
shivering in patients undergoing cesarean section. The aim of this
meta-analysis was to evaluate whether intrathecal DEX could prevent shivering
in cesarean section after spinal anesthesia.
Methods: We searched PubMed, Embase, Web of Science, and the Cochrane
Library for randomized controlled trials (RCTs) comparing intrathecal DEX in
cesarean section after spinal anesthesia with placebo and reporting on
shivering, postoperative nausea and vomiting (PONV), hypotension, and
bradycardia. Trial sequential analysis (TSA) was also carried out for RCTs
comparing DEX with placebo. This meta-analysis has been registered on PROSPERO,
and the registration number is CRD42017071640.
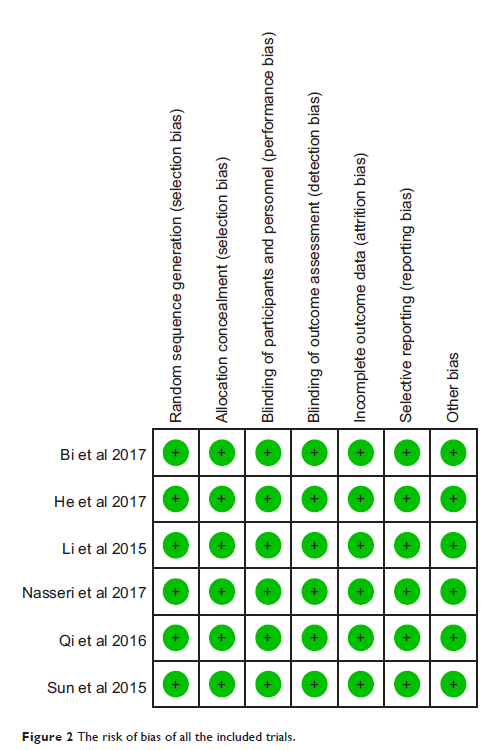
Results: Six randomized clinical trials comparing 360 patients were
included in this study. Compared with placebo, intrathecal DEX significantly
reduced the incidence of shivering (risk ratio [RR]=0.40; 95% CI [0.26,
0.62]; P <0.0001). No significant
difference was found in the incidence of PONV (RR=1.34; 95% CI [0.82,
2.18]; P =0.24), hypotension (RR=1.09; 95%
CI [0.84, 1.42]; P =0.50), or
bradycardia (RR=1.55; 95% CI [0.54, 4.42]; P =0.42).
However, no firm conclusions can be made based on the results of all outcomes
according to the TSA.
Conclusion: This meta-analysis found that intrathecal DEX could prevent
shivering in cesarean section after spinal anesthesia and did not induce PONV,
hypotension, or bradycardia. However, firm conclusions cannot be made until
more studies are conducted.
Keywords: dexmedetomidine, cesarean section, spinal anesthesia,
meta-analysis, trial sequential analysis, adverse effect