108552
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

人工控制的可降解纳米粒子可作为磁共振成像造影剂的替代及用于编程癌症治疗
Authors Yun T, Liu Y, Yi S, Jia Q, Liu Y, Zhou J
Received 1 August 2018
Accepted for publication 23 September 2018
Published 24 October 2018 Volume 2018:13 Pages 6647—6659
DOI https://doi.org/10.2147/IJN.S182206
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Background: Utilizing the permeability enhancement and irreversible
biomolecule denaturation caused by hyperthermia, photothermal-chemo synergistic
therapy has shown great potential in clinical cancer treatment.
Purpose: The objective of this study was to provide a novel controlled drug
release method to improve the efficiency of photothermal-chemo synergistic
therapy.
Patients and
methods: HCT116 tumor-bearing mice were
selected as modal for the study of cancer theranostics efficiency. The T2 to T1
magnetic resonance imaging contrast switch was studied in vivo. Analyses of the
tumor growth of mice were carried out to evaluate the tumor therapy efficiency.
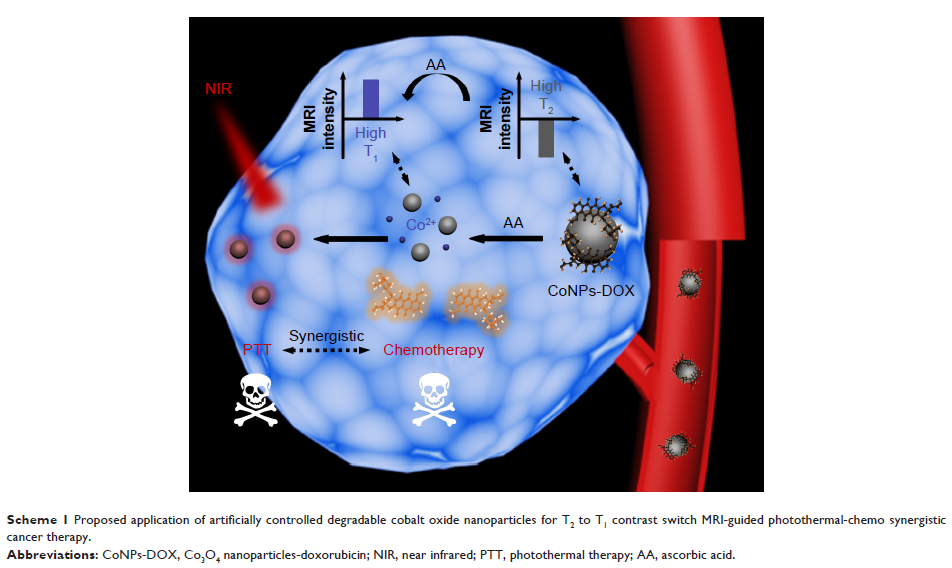
Results: we developed novel artificially controlled degradable Co3O4 nanoparticles and
explored their potential in drug delivery/release. In the presence of ascorbic
acid (AA), the designed nanomaterials can be degraded via a redox process and
hence release the loaded drugs. Importantly, the AA, in the lack of
l-gulonolactone oxidase, cannot be synthesized in the body of typical mammal
including human, which suggested that the degradation process can be controlled
artificially. Moreover, the obtained nanoparticles have outstanding
photothermal conversion efficiency and their degradation can also result in an
magnetic resonance imaging contrast enhancement switch from T2 to T1, which benefits the cancer theranostics.
Conclusion: Our results illustrated that the artificially controlled degradable
nanoparticles can serve as an alternative candidate for controllable drug
release as well as a platform for highly efficient photothermal-chemo
synergistic cancer theranostics.
Keywords: photothermal, degradable, controllable drug release, synergistic
therapy, MRI