108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

胸腺素 α1 通过抑制 STAT3-MMP2 信号通路遏制 PD-L1 高表达非小细胞肺癌细胞的迁移和侵袭
Authors Bo C, Wu Q, Zhao H, Li X, Zhou Q
Received 22 June 2018
Accepted for publication 15 September 2018
Published 23 October 2018 Volume 2018:11 Pages 7255—7270
DOI https://doi.org/10.2147/OTT.S177943
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Justinn Cochran
Peer reviewer comments 5
Editor who approved publication: Dr Leo Jen-Liang Su
Background: Thymosin α1 (Tα1) is one of the most commonly used immunomodulators for
metastatic non-small-cell lung cancer (NSCLC) patients in many countries.
Despite the identification of the direct suppression on cancer cell
proliferation, little is known about its effect on metastasis and metastasis-related
signaling such as matrix metalloproteinases (MMPs) and programmed cell death
ligand 1 (PD-L1).
Materials and
methods: NSCLC cells with
distinguishing PD-L1 expression levels were treated with Tα1. siRNAs were used
to knockdown PD-L1. Cell migration and invasion abilities were evaluated by
wound-healing and transwell assays. The xenograft model by BALB/c nude mice was
constructed to test the inhibitory effect of Tα1 on metastasis in vivo. The
expression levels of metastasis-related signaling pathways and key molecules
were assessed by Western blot (WB) and quantitative reverse transcriptase PCR
(qRT-PCR).
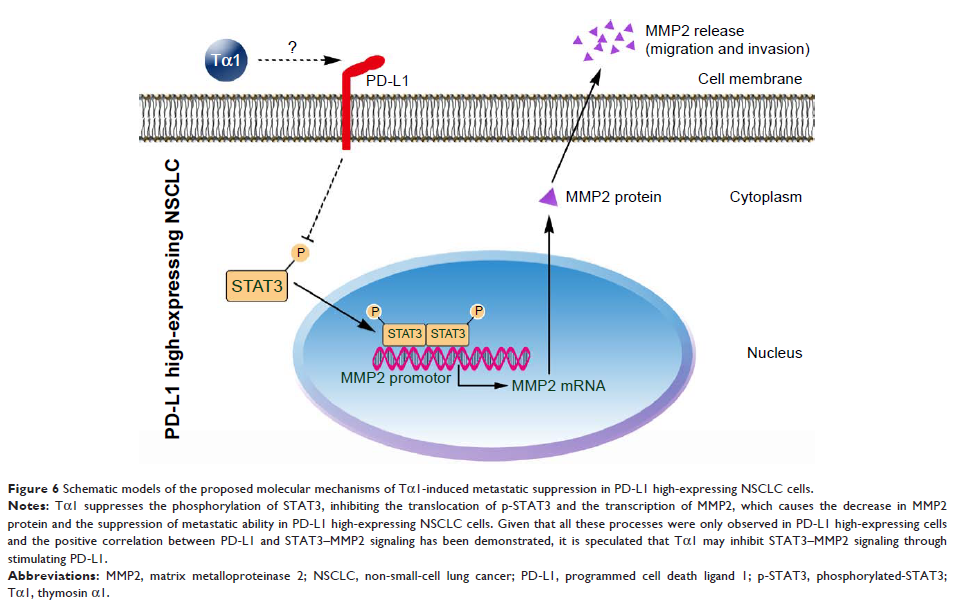
Results: Tα1 significantly suppressed cell migration and invasion in PD-L1
high-expressing H1299, NL9980, and L9981 cells but not in PD-L1 low-expressing
A549 or SPC-A-1 cells. This difference was demonstrated by mouse model in vivo
as well. Knocking down of PD-L1 significantly impaired the inhibition of cell
migration and invasion caused by Tα1 treating in PD-L1 high-expressing cells.
Besides, Tα1 inhibited the activation and translocation of STAT3 and the
expression of MMP2 in PD-L1 high-expressing NSCLC cells. Moreover, the
treatment of STAT3 activator colivelin could partly reverse the Tα1-induced
MMP2 suppression and the migration phenotype.
Conclusion: Tα1 significantly suppresses migration and invasion in PD-L1
high-expressing NSCLC cells compared with PD-L1 low-expressing NSCLC cells in
vitro and in vivo, through the downregulation of STAT3–MMP2 signaling. These
different responses to Tα1, together with the depiction of Tα1-induced
signaling changes, suggest a potential benefit of Tα1 for PD-L1-positive NSCLC
patients, enlightening the combination of Tα1 with target therapy or immune
checkpoint inhibitors.
Keywords: matrix metalloproteinase 2, non-small-cell lung cancer, programmed cell
death ligand 1, STAT3, thymosin α1