108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

血管新生阻滞治疗胃癌的疗效系统评价和荟萃分析
Authors Bai ZG, Zhang ZT
Received 28 March 2018
Accepted for publication 1 June 2018
Published 17 October 2018 Volume 2018:11 Pages 7077—7087
DOI https://doi.org/10.2147/OTT.S169484
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 4
Editor who approved publication: Dr Carlos E Vigil
Introduction: To date, anti-vascular endothelial growth factor (VEGF) monoclonal
antibody (mAb, bevacizumab), anti-VEGF receptor mAb (ramucirumab) and selective
vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors
(sunitinib, sorafenib and apatinib) have been tested in the clinical trials.
Materials and
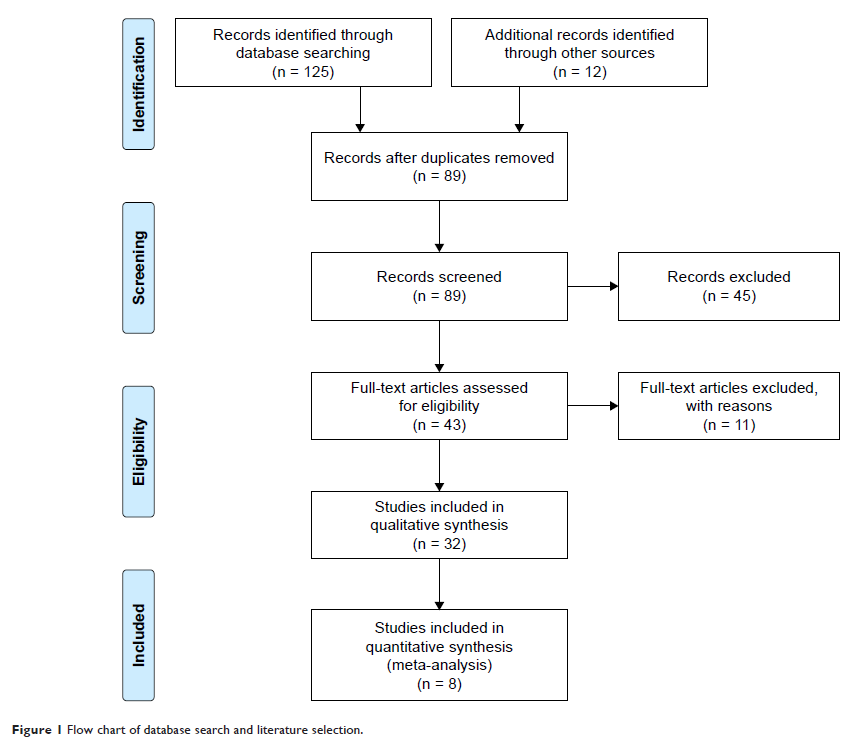
methods: In the current study, results of 32
clinical trials (24 Phase I or II, 8 Phase III) were systematically reviewed
and meta-analysis was performed in 8 Phase III trial results.
Results: It was found that median overall survival (OS) time and progression-free
survival (PFS) time were significantly longer in the patients treated with
antiangiogenic reagents compared to that in the patients with placebo when all
of 8 Phase III clinical trials were analyzed together (OS: odds ratio = 0.805,
95% CI: 0.719–0.901, P < 0.001;
PFS: odds ratio = 0.719, 95% CI: 0.533–969, P =
0.030).
Conclusion: Meta-analysis on bevacizumab (4 out 8 Phase III trials) indicated
that neither OS nor PFS was significantly different between the groups treated
with bevacizumab or placebo with or without combination of other chemotherapeutic
reagents (OS: odds ratio = 0.909, 95% CI: 0.780–1.059, P = 0.221; PFS: odds ratio =
0.985, 95% CI: 0.865–1.122, P = 0.826). By
contrast, meta-analysis on ramucirumab (3 out of 8 Phase III trials) revealed
that ramucirumab was significantly favored in the treatment of gastric cancer
with significant different OS between the two groups (odds ratio = 0.720, 95%
CI: 0.604–0.858, P < 0.001).
In addition, patients treated with VEGF or VEGFR blockers had higher morbidity
of hypertension and neutropenia, but lower risk of side effects of vomiting and
anemia. These findings suggest that addition of antiangiogenesis reagents,
especially anti-VEGFR-mAb, to the first- or second-line chemotherapy could
prolong patient’s OS and PFS time in the advanced or metastatic gastric cancer.
Keywords: anti-VEGF monoclonal antibody, anti-VEGF receptor mAb, VEGFR
tyrosine kinase inhibitors, Phase III trial, overall survival, progression-free
survival, chemotherapy