108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

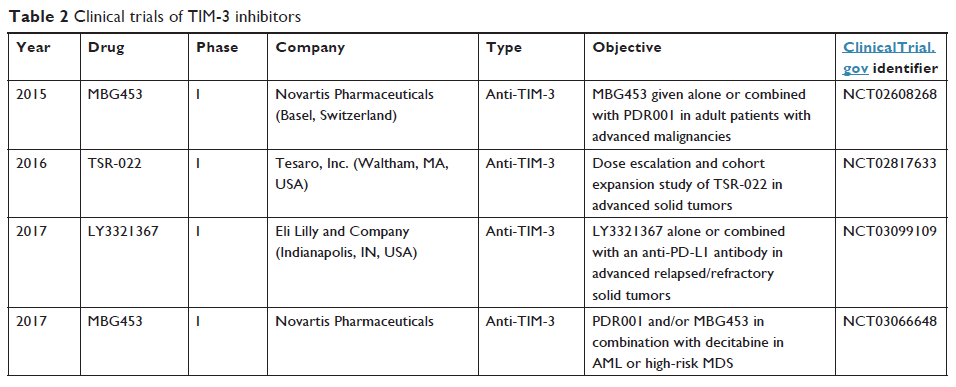
TIM-3 是癌症免疫疗法的有希望目标
Authors He Y, Cao J, Zhao C, Li X, Zhou C, Hirsch FR
Received 7 April 2018
Accepted for publication 9 August 2018
Published 16 October 2018 Volume 2018:11 Pages 7005—7009
DOI https://doi.org/10.2147/OTT.S170385
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Abstract: Patients with malignant tumor treated with immunotherapy have
received significant clinical benefits over the years. Immune checkpoint
blocking agents, such as anti-cytotoxic T-lymphocyte-associated protein-4
(anti-CTLA-4) and anti-programmed cell death protein-1 (anti-PD-1) monoclonal
antibodies, have produced impressive clinical results in different types of
cancer. T-cell immunoglobulin and mucin domain-3 (TIM-3), another immune
checkpoint, could inhibit cancer immunity. Recent studies have highlighted that
TIM-3 has an important role to play in T-cell exhaustion and correlates with
the outcome of anti-PD-1 therapy. Targeting TIM-3 might be a promising approach
for cancer immunotherapy. Here, we review the role of TIM-3 in cancer and
clinical trials with TIM-3 inhibitors.
Keywords: immune checkpoint, clinical trial, cancer immunotherapy, T-cell
immunoglobulin and mucin domain-3 (TIM-3)